Kosin Med J.
2023 Jun;38(2):87-97. 10.7180/kmj.23.125.
Are you ready to accompany autosomal dominant polycystic kidney disease patients in their treatment journey? Real practice for selecting rapid progressors and treatment with tolvaptan
- Affiliations
-
- 1Department of Internal Medicine, Kosin University College of Medicine, Busan, Korea
- 2Department of Internal Medicine, SMG-SNU Boramae Medical Center, Seoul, Korea
- KMID: 2543328
- DOI: http://doi.org/10.7180/kmj.23.125
Abstract
- Tolvaptan treatment is costly, often accompanied by aquaresis-related adverse events, and requires careful monitoring by medical staff due to the possibility of hepatotoxicity. Nevertheless, it is the only disease-modifying drug to date that has been shown to successfully delay renal replacement therapy. For more patients to receive proper treatment, medical doctors, the rest of the medical team, and the patient must all work together. This paper reviews parameters that can help identify rapid autosomal dominant polycystic kidney disease progressors, who are the target of tolvaptan therapy. It is expected that these parameters will help nephrologists learn practical prescription methods and identify patients who can benefit from tolvaptan treatment. Although several strategies can be used to find rapid progressors, the present review focuses on a practical method to identify rapid progressors according to the presence or absence of evidence and the factors associated with rapid progression based on the Mayo image classification.
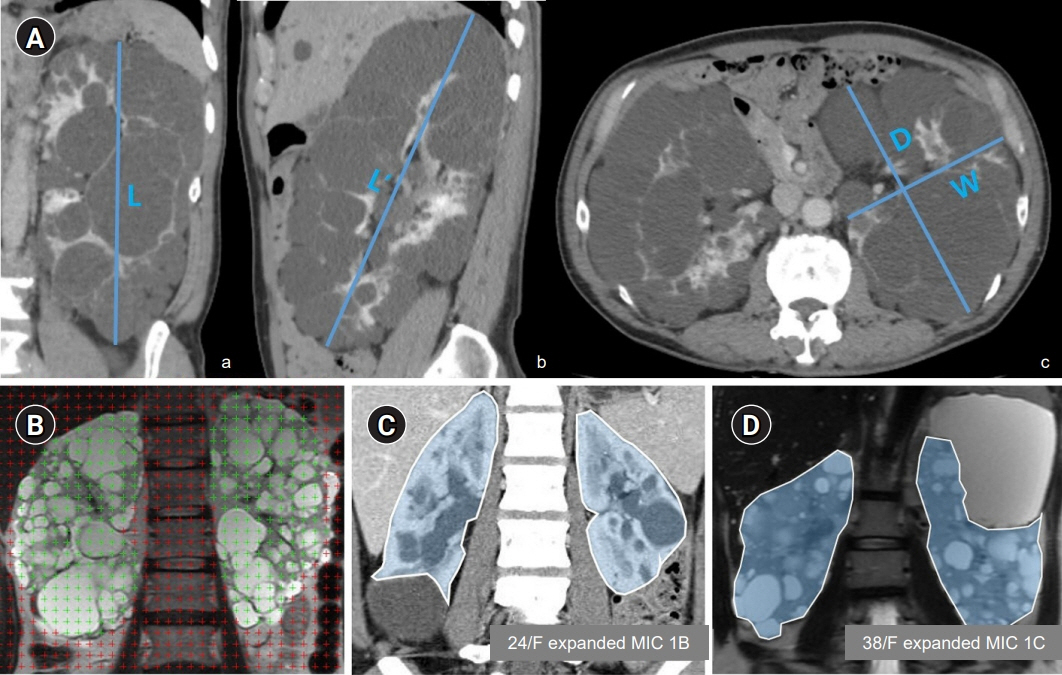
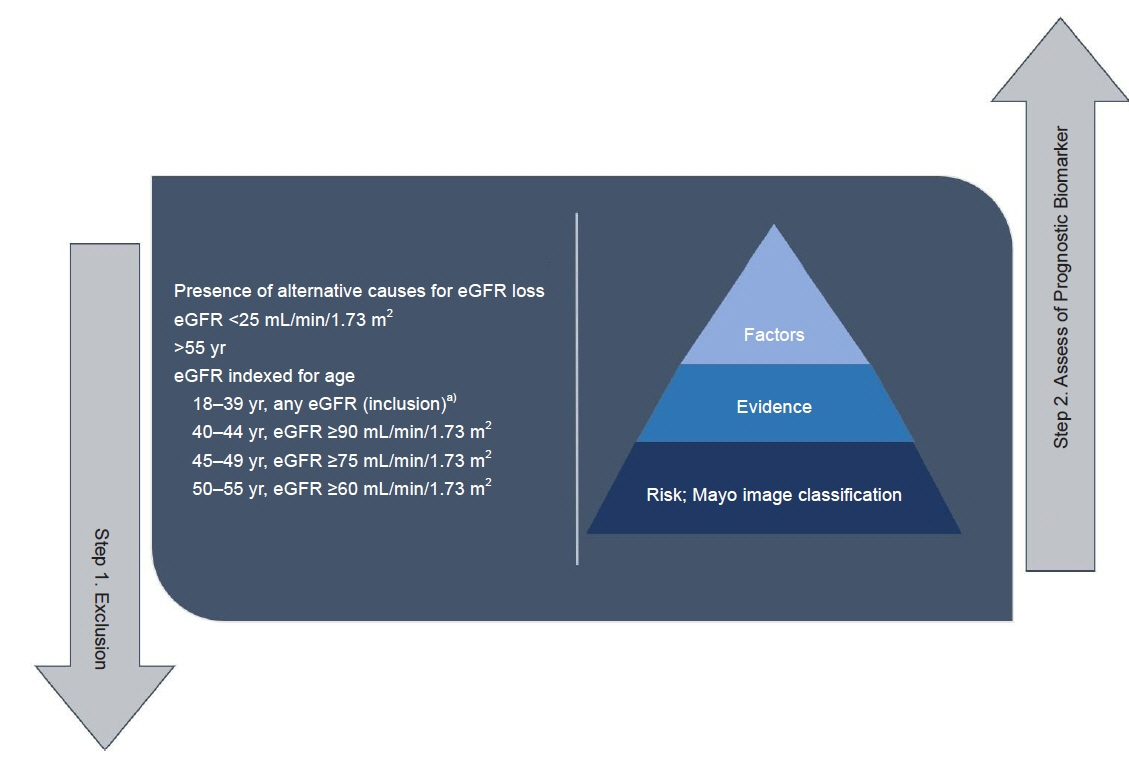
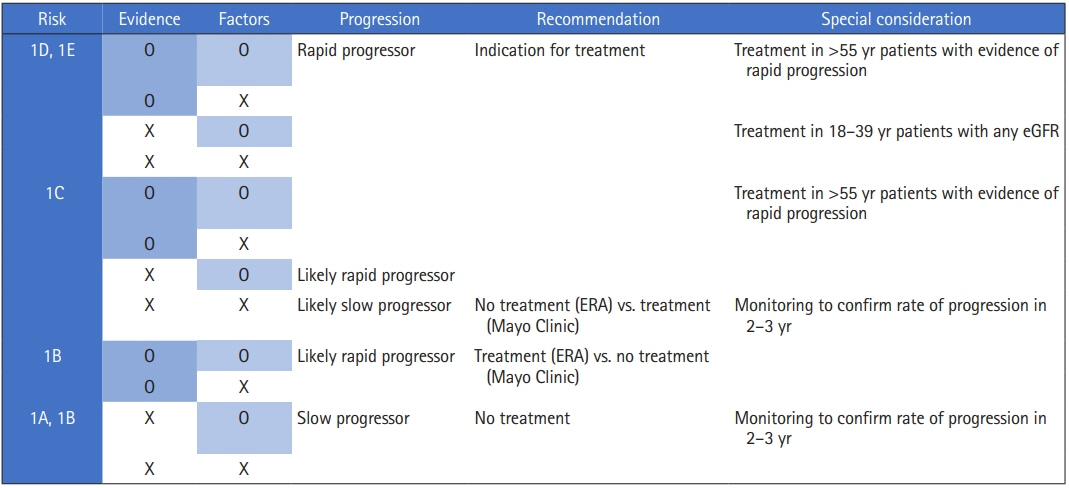
Figure
Reference
-
References
1. Gabow PA. Autosomal dominant polycystic kidney disease. N Engl J Med. 1993; 329:332–42.2. Chapman AB, Johnson A, Gabow PA, Schrier RW. The renin-angiotensin-aldosterone system and autosomal dominant polycystic kidney disease. N Engl J Med. 1990; 323:1091–6.3. Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, et al. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012; 367:2407–18.4. Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Perrone RD, Koch G, et al. Tolvaptan in later-stage autosomal dominant polycystic kidney disease. N Engl J Med. 2017; 377:1930–42.5. Barnawi RA, Attar RZ, Alfaer SS, Safdar OY. Is the light at the end of the tunnel nigh? A review of ADPKD focusing on the burden of disease and tolvaptan as a new treatment. Int J Nephrol Renovasc Dis. 2018; 11:53–67.6. McBride L, Wilkinson C, Jesudason S. Management of Autosomal Dominant Polycystic Kidney Disease (ADPKD) during pregnancy: risks and challenges. Int J Womens Health. 2020; 12:409–22.7. Liu F, Feng C, Shen H, Fu H, Mao J. Tolvaptan in pediatric autosomal dominant polycystic kidney disease: from here to where? Kidney Dis (Basel). 2021; 7:343–9.8. Chebib FT, Torres VE. Assessing risk of rapid progression in autosomal dominant polycystic kidney disease and special considerations for disease-modifying therapy. Am J Kidney Dis. 2021; 78:282–92.9. Spithoven EM, Kramer A, Meijer E, Orskov B, Wanner C, Caskey F, et al. Analysis of data from the ERA-EDTA Registry indicates that conventional treatments for chronic kidney disease do not reduce the need for renal replacement therapy in autosomal dominant polycystic kidney disease. Kidney Int. 2014; 86:1244–52.10. Cornec-Le Gall E, Audrezet MP, Rousseau A, Hourmant M, Renaudineau E, Charasse C, et al. The PROPKD score: a new algorithm to predict renal survival in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2016; 27:942–51.11. Irazabal MV, Rangel LJ, Bergstralh EJ, Osborn SL, Harmon AJ, Sundsbak JL, et al. Imaging classification of autosomal dominant polycystic kidney disease: a simple model for selecting patients for clinical trials. J Am Soc Nephrol. 2015; 26:160–72.12. Park HC, Hong Y, Yeon JH, Ryu H, Kim YC, Lee J, et al. Mayo imaging classification is a good predictor of rapid progress among Korean patients with autosomal dominant polycystic kidney disease: results from the KNOW-CKD study. Kidney Res Clin Pract. 2022; 41:432–41.13. Yu AS, Shen C, Landsittel DP, Grantham JJ, Cook LT, Torres VE, et al. Long-term trajectory of kidney function in autosomal-dominant polycystic kidney disease. Kidney Int. 2019; 95:1253–61.14. Soroka S, Alam A, Bevilacqua M, Girard LP, Komenda P, Loertscher R, et al. Updated Canadian expert consensus on assessing risk of disease progression and pharmacological management of autosomal dominant polycystic kidney disease. Can J Kidney Health Dis. 2018; 5:2054358118801589.15. Muller RU, Messchendorp AL, Birn H, Capasso G, Cornec-Le Gall E, Devuyst O, et al. An update on the use of tolvaptan for autosomal dominant polycystic kidney disease: consensus statement on behalf of the ERA Working Group on Inherited Kidney Disorders, the European Rare Kidney Disease Reference Network and Polycystic Kidney Disease International. Nephrol Dial Transplant. 2022; 37:825–39.16. Bae KT, Shi T, Tao C, Yu AS, Torres VE, Perrone RD, et al. Expanded imaging classification of autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2020; 31:1640–51.17. King BF, Reed JE, Bergstralh EJ, Sheedy PF 2nd, Torres VE. Quantification and longitudinal trends of kidney, renal cyst, and renal parenchyma volumes in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2000; 11:1505–11.18. Kistler AD, Poster D, Krauer F, Weishaupt D, Raina S, Senn O, et al. Increases in kidney volume in autosomal dominant polycystic kidney disease can be detected within 6 months. Kidney Int. 2009; 75:235–41.19. Bae KT, Commean PK, Lee J. Volumetric measurement of renal cysts and parenchyma using MRI: phantoms and patients with polycystic kidney disease. J Comput Assist Tomogr. 2000; 24:614–9.20. Magistroni R, Corsi C, Marti T, Torra R. A review of the imaging techniques for measuring kidney and cyst volume in establishing autosomal dominant polycystic kidney disease progression. Am J Nephrol. 2018; 48:67–78.21. Horie S, Mochizuki T, Muto S, Hanaoka K, Fukushima Y, Narita I, et al. Evidence-based clinical practice guidelines for polycystic kidney disease 2014. Clin Exp Nephrol. 2016; 20:493–509.22. Barua M, Cil O, Paterson AD, Wang K, He N, Dicks E, et al. Family history of renal disease severity predicts the mutated gene in ADPKD. J Am Soc Nephrol. 2009; 20:1833–8.23. Lanktree MB, Guiard E, Li W, Akbari P, Haghighi A, Iliuta IA, et al. Intrafamilial variability of ADPKD. Kidney Int Rep. 2019; 4:995–1003.24. Chebib FT, Perrone RD, Chapman AB, Dahl NK, Harris PC, Mrug M, et al. A practical guide for treatment of rapidly progressive ADPKD with tolvaptan. J Am Soc Nephrol. 2018; 29:2458–70.25. Wulfmeyer VC, Auber B, Haller H, Schmitt R. Comparison of different selection strategies for tolvaptan eligibility among autosomal dominant polycystic kidney disease patients. Am J Nephrol. 2019; 50:281–90.26. Radhakrishnan Y, Duriseti P, Chebib FT. Management of autosomal dominant polycystic kidney disease in the era of disease-modifying treatment options. Kidney Res Clin Pract. 2022; 41:422–31.27. Zhou JX, Torres VE. Autosomal dominant polycystic kidney disease therapies on the horizon. Adv Kidney Dis Health. 2023; 30:245–60.28. Devuyst O, Torres VE. Osmoregulation, vasopressin, and cAMP signaling in autosomal dominant polycystic kidney disease. Curr Opin Nephrol Hypertens. 2013; 22:459–70.29. Shoaf SE, Chapman AB, Torres VE, Ouyang J, Czerwiec FS. Pharmacokinetics and pharmacodynamics of tolvaptan in autosomal dominant polycystic kidney disease: phase 2 trials for dose selection in the pivotal phase 3 trial. J Clin Pharmacol. 2017; 57:906–17.30. Watkins PB, Lewis JH, Kaplowitz N, Alpers DH, Blais JD, Smotzer DM, et al. Clinical pattern of tolvaptan-associated liver injury in subjects with autosomal dominant polycystic kidney disease: analysis of clinical trials database. Drug Saf. 2015; 38:1103–13.31. Muto S, Kawano H, Higashihara E, Narita I, Ubara Y, Matsuzaki T, et al. The effect of tolvaptan on autosomal dominant polycystic kidney disease patients: a subgroup analysis of the Japanese patient subset from TEMPO 3:4 trial. Clin Exp Nephrol. 2015; 19:867–77.32. Akihisa T, Manabe S, Kataoka H, Makabe S, Yoshida R, Ushio Y, et al. Dose-dependent effect of tolvaptan on renal prognosis in patients with autosomal dominant polycystic kidney disease. Kidney360. 2021; 2:1148–51.33. Devuyst O, Chapman AB, Gansevoort RT, Higashihara E, Perrone RD, Torres VE, et al. Urine osmolality, response to tolvaptan, and outcome in autosomal dominant polycystic kidney disease: results from the TEMPO 3:4 trial. J Am Soc Nephrol. 2017; 28:1592–602.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Management of autosomal dominant polycystic kidney disease in the era of disease-modifying treatment options
- Tolvaptan: a possible preemptive treatment option in children with autosomal dominant polycystic kidney disease?
- Recent updates in therapeutic approach using tolvaptan for autosomal dominant polycystic kidney disease
- Long-term Tolvaptan Treatment of Autosomal Dominant Polycystic Kidney Disease in Korea
- Clinical Implication of Tolvaptan in Patients with Autosomal Dominant Polycystic Kidney Disease