Ann Hepatobiliary Pancreat Surg.
2019 Feb;23(1):46-55. 10.14701/ahbps.2019.23.1.46.
Validation of the eighth edition of the American Joint Committee on Cancer staging system and proposal of an improved staging system for pancreatic ductal adenocarcinoma
- Affiliations
-
- 1Division of Hepato-Biliary and Pancreatic Surgery, Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. drksc@amc.seoul.kr
- 2Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 2439045
- DOI: http://doi.org/10.14701/ahbps.2019.23.1.46
Abstract
- BACKGROUNDS/AIMS
This study aimed to validate the eighth edition of the American Joint Committee on Cancer (AJCC) staging system for pancreatic adenocarcinoma and to propose an improved staging system for this disease.
METHODS
Between 2000 and 2014, 1656 patients underwent surgical resection for pancreatic ductal adenocarcinoma at Asan Medical Center, Seoul, South Korea. The 1169 patients included in this study were recategorized according to the eighth edition of the AJCC staging system. Patients were also categorized according to a new staging system, based on tumor size and number of metastatic lymph nodes.
RESULTS
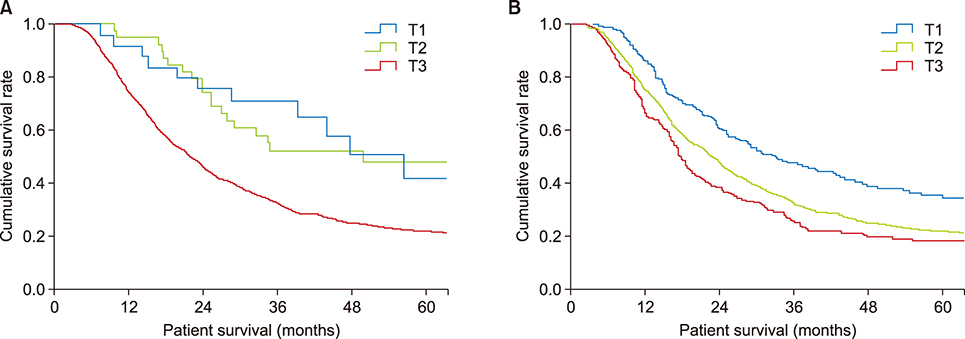
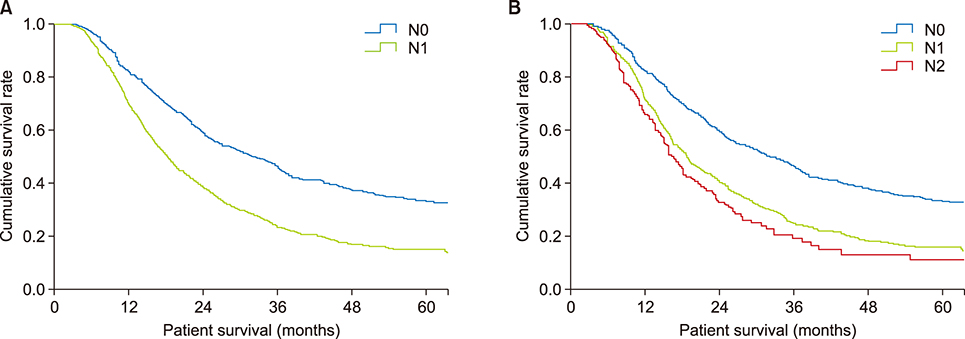
The seventh edition of the AJCC staging system categorized 93.7% of patients as having stage T3 tumors. Stages were distributed more evenly with the eighth edition. In the N0 group, classification according to the seventh edition showed no statistically significant differences in survival rate between patients with T1 and T2 (p=0.717) and with IA and IB (p=0.717) tumors. Survival rates classified according to the eighth edition differed significantly for all pairs of T stages (p < 0.05). With both editions, N stages showed statistically significant differences (p < 0.05). Reanalysis showed that a staging system using a tumor size ≥3 cm and ≥1 metastatic lymph nodes was more predictive of survival rates.
CONCLUSIONS
Compared with the seventh edition, the eighth edition of the AJCC staging system for pancreatic adenocarcinoma showed a more even distribution in T stage but marginal differences in other stages. The proposed system, using tumor size and number of metastatic lymph nodes, was better at predicting survival.
Keyword
MeSH Terms
Figure
Reference
-
1. Conlon KC, Klimstra DS, Brennan MF. Long-term survival after curative resection for pancreatic ductal adenocarcinoma. Clinicopathologic analysis of 5-year survivors. Ann Surg. 1996; 223:273–279.2. Sener SF, Fremgen A, Menck HR, Winchester DP. Pancreatic cancer: a report of treatment and survival trends for 100,313 patients diagnosed from 1985-1995, using the National Cancer Database. J Am Coll Surg. 1999; 189:1–7.3. Kindler HL. Pancreatic cancer: an update. Curr Oncol Rep. 2007; 9:170–176.
Article4. Geer RJ, Brennan MF. Prognostic indicators for survival after resection of pancreatic adenocarcinoma. Am J Surg. 1993; 165:68–72. discussion 72-73.
Article5. Schnelldorfer T, Ware AL, Sarr MG, Smyrk TC, Zhang L, Qin R, et al. Long-term survival after pancreatoduodenectomy for pancreatic adenocarcinoma: is cure possible? Ann Surg. 2008; 247:456–462.6. Nitecki SS, Sarr MG, Colby TV, van Heerden JA. Long-term survival after resection for ductal adenocarcinoma of the pancreas. Is it really improving? Ann Surg. 1995; 221:59–66.
Article7. Cleary SP, Gryfe R, Guindi M, Greig P, Smith L, Mackenzie R, et al. Prognostic factors in resected pancreatic adenocarcinoma: analysis of actual 5-year survivors. J Am Coll Surg. 2004; 198:722–731.8. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010; 17:1471–1474.
Article9. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The eighth edition AJCC Cancer Staging Manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017; 67:93–99.
Article10. Varadhachary GR, Tamm EP, Abbruzzese JL, Xiong HQ, Crane CH, Wang H, et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol. 2006; 13:1035–1046.
Article11. Bilimoria KY, Bentrem DJ, Ko CY, Tomlinson JS, Stewart AK, Winchester DP, et al. Multimodality therapy for pancreatic cancer in the U.S. : utilization, outcomes, and the effect of hospital volume. Cancer. 2007; 110:1227–1234.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Updated guidelines on the preoperative staging of thyroid cancer
- Assessment of the Prognostic Staging System of American Joint Committee on Cancer 8th Edition for Breast Cancer: Comparisons with the Conventional Anatomic Staging System
- A Comparison of Fifth-Edition and Sixth-Edition American Joint Committee Staging System for Sinonasal Squamous Cell Carcinoma
- Introduction of 7th AJCC TNM Staging for Hepatobiliary, Pancreatic Ampulla of Vater, Exocrine and Endocrine Cancers
- Introduction of 7th AJCC Cancer Staging: Gallbladder, Perihilar Bile Duct and Distal Common Bile Duct