J Breast Cancer.
2020 Feb;23(1):59-68. 10.4048/jbc.2020.23.e9.
Assessment of the Prognostic Staging System of American Joint Committee on Cancer 8th Edition for Breast Cancer: Comparisons with the Conventional Anatomic Staging System
- Affiliations
-
- 1Department of Surgery, Yonsei University College of Medicine, Seoul, Korea. hyungseokpark.md@gmail.com
- KMID: 2470882
- DOI: http://doi.org/10.4048/jbc.2020.23.e9
Abstract
- PURPOSE
The 8th edition of the American Joint Committee on Cancer (AJCC) staging manual introduced a new prognostic staging system for breast cancer. This study aimed to evaluate the changes in staging distribution and predictive power of the new staging system.
METHODS
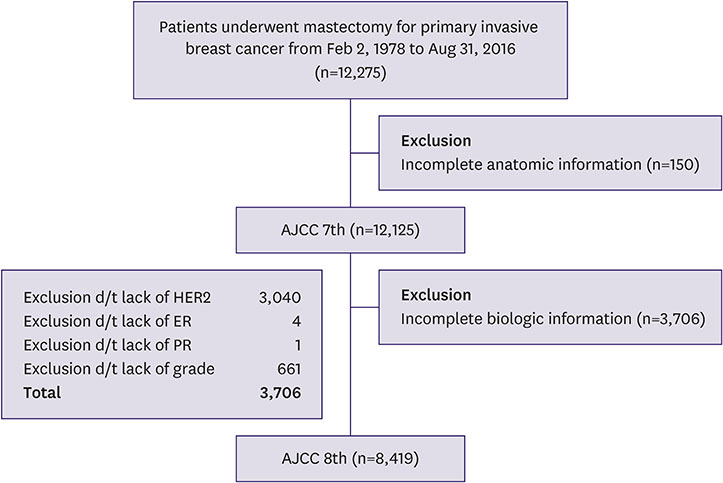
Of the 12,275 patients with breast cancer identified from the Severance Breast Cancer Registry who underwent surgery between 1978 and 2016, 12,125 patients met the inclusion criteria.
RESULTS
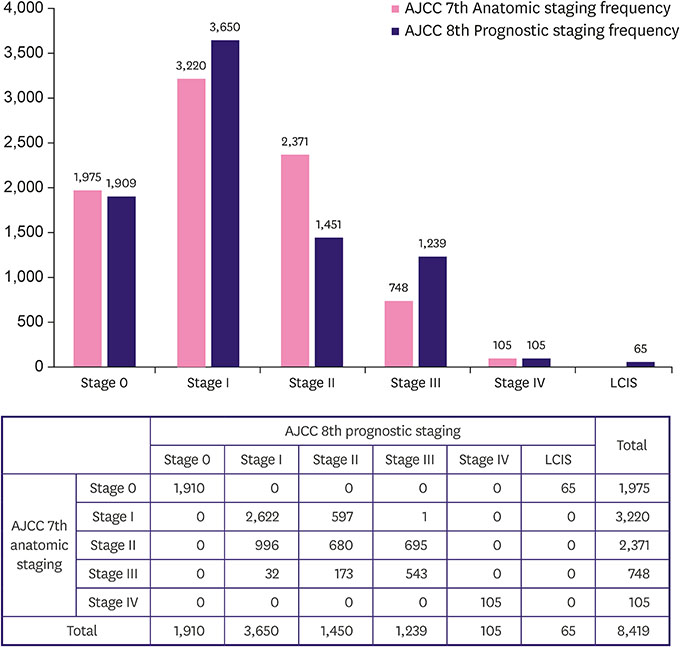
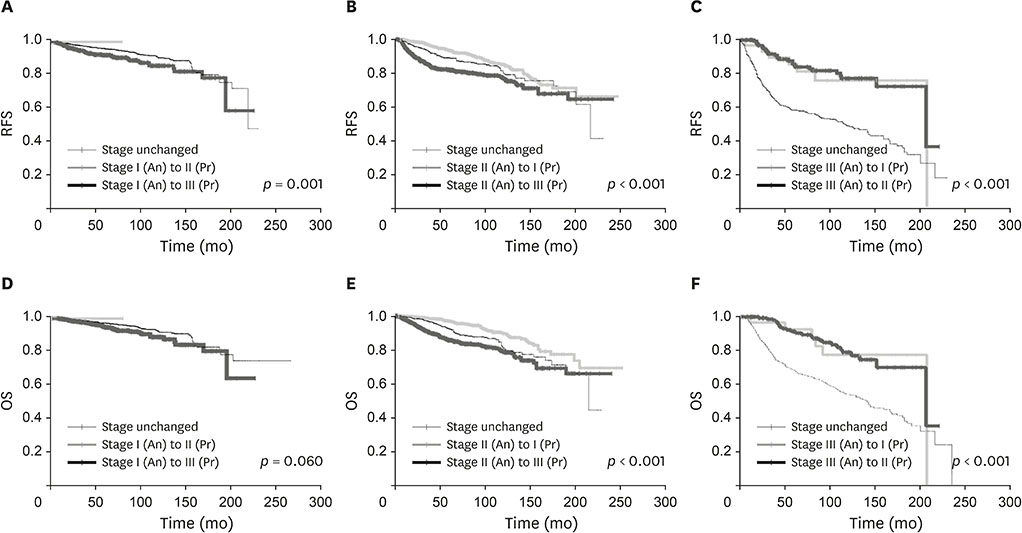
In both the 7th and 8th staging systems, stage I patients constituted the largest proportion (38.2% and 48.4%). Migration from the 7th to 8th edition of the AJCC manual resulted in a decrease in stage II population and an increase in stage I and III populations. A total of 1,293 (15.4%) patients were upstaged, and 1,201 (14.3%) were downstaged. Downstaged patients had better recurrence-free and overall survival (p < 0.001). Pathologic complete response after neoadjuvant therapy showed good prognosis as p stage 0, and yp stages I and III showed poorer outcomes than the same p stage (p < 0.001).
CONCLUSIONS
Staging migrations are common in early breast cancer under the prognostic staging system. The prognostic staging system of the 8th edition of the AJCC manual discriminates survival outcomes better than the anatomical staging system of the 7th edition of the AJCC manual.
Keyword
Figure
Reference
-
1. Weiss A, Chavez-MacGregor M, Lichtensztajn DY, Yi M, Tadros A, Hortobagyi GN, et al. Validation study of the American Joint Committee on Cancer eighth edition prognostic stage compared with the anatomic stage in breast cancer. JAMA Oncol. 2018; 4:203–209.
Article2. Amin MB, Edge SB. AJCC Cancer Staging Manual. New York (NY): Springer;2017.3. Gospodarowicz MK, Brierley JD, Wittekind C. TNM Classification of Malignant Tumours. Chichester: Wiley;2017.4. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The eighth edition AJCC Cancer Staging Manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017; 67:93–99.
Article5. Harris LN, Ismaila N, McShane LM, Andre F, Collyar DE, Gonzalez-Angulo AM, et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016; 34:1134–1150.
Article6. Wang M, Chen H, Wu K, Ding A, Zhang M, Zhang P. Evaluation of the prognostic stage in the 8th edition of the American Joint Committee on Cancer in locally advanced breast cancer: an analysis based on SEER 18 database. Breast. 2018; 37:56–63.
Article7. Wong RX, Wong FY, Lim J, Lian WX, Yap YS. Validation of the AJCC 8th prognostic system for breast cancer in an Asian healthcare setting. Breast. 2018; 40:38–44.
Article8. Giuliano AE, Connolly JL, Edge SB, Mittendorf EA, Rugo HS, Solin LJ, et al. Breast cancer-major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017; 67:290–303.
Article9. Park S, Park BW, Kim TH, Jeon CW, Kang HS, Choi JE, et al. Lack of either estrogen or progesterone receptor expression is associated with poor survival outcome among luminal A breast cancer subtype. Ann Surg Oncol. 2013; 20:1505–1513.
Article10. Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010; 28:2784–2795.
Article11. Park S, Park HS, Koo JS, Yang WI, Kim SI, Park BW. Breast cancers presenting luminal B subtype features show higher discordant human epidermal growth factor receptor 2 results between immunohistochemistry and fluorescence in situ hybridization. Cancer. 2012; 118:914–923.
Article12. Ibis K, Ozkurt S, Kucucuk S, Yavuz E, Saip P. Comparison of pathological prognostic stage and anatomic stage groups according to the updated version of the American Joint Committee on Cancer (AJCC) breast cancer staging 8th edition. Med Sci Monit. 2018; 24:3637–3643.
Article13. Hu H, Wei W, Yi X, Xin L, Liu Y. A retrospective analysis of clinical utility of AJCC 8th edition cancer staging system for breast cancer. World J Oncol. 2017; 8:71–75.
Article14. Abdel-Rahman O. Validation of the 8th AJCC prognostic staging system for breast cancer in a population-based setting. Breast Cancer Res Treat. 2018; 168:269–275.
Article15. von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012; 30:1796–1804.
Article16. Campbell JI, Yau C, Krass P, Moore D, Carey LA, Au A, et al. Comparison of residual cancer burden, American Joint Committee on Cancer staging and pathologic complete response in breast cancer after neoadjuvant chemotherapy: results from the I-SPY 1 TRIAL (CALGB 150007/150012; ACRIN 6657). Breast Cancer Res Treat. 2017; 165:181–191.
Article17. Mazouni C, Peintinger F, Wan-Kau S, Andre F, Gonzalez-Angulo AM, Symmans WF, et al. Residual ductal carcinoma in situ in patients with complete eradication of invasive breast cancer after neoadjuvant chemotherapy does not adversely affect patient outcome. J Clin Oncol. 2007; 25:2650–2655.
Article18. Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014; 384:164–172.
Article19. Jones RL, Lakhani SR, Ring AE, Ashley S, Walsh G, Smith IE. Pathological complete response and residual DCIS following neoadjuvant chemotherapy for breast carcinoma. Br J Cancer. 2006; 94:358–362.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prognostic Validation of the American Joint Committee on Cancer 8th Staging System in 24,014 Korean Patients with Breast Cancer
- Introduction of a New Staging System of Breast Cancer for Radiologists: An Emphasis on the Prognostic Stage
- Development of an Excel Program for the Updated Eighth American Joint Committee on Cancer Breast Cancer Staging System
- Prognostic Predictability of American Joint Committee on Cancer 8th Staging System for Perihilar Cholangiocarcinoma: Limited Improvement Compared with the 7th Staging System
- Clinicopathologic Implication of New AJCC 8(th) Staging Classification in the Stomach Cancer