Clin Exp Vaccine Res.
2019 Jan;8(1):43-53. 10.7774/cevr.2019.8.1.43.
Vaccine containing G protein fragment and recombinant baculovirus expressing M2 protein induces protective immunity to respiratory syncytial virus
- Affiliations
-
- 1Graduate School of Pharmaceutical Sciences, Ewha Womans University, Seoul, Korea. TCELL@EWHA.AC.KR
- KMID: 2438962
- DOI: http://doi.org/10.7774/cevr.2019.8.1.43
Abstract
- PURPOSE
Respiratory syncytial virus (RSV) can cause serious respiratory illnesses such as pneumonia, asthma, and bronchiolitis in infants and elderly or immunocompromised individuals. An RSV vaccine has yet to be developed; only prophylactic anti-RSV antibody is commercially available. So, we investigated whether our vaccine candidate is able to induce type 1 CD4+ T helper (Th1), CD8+ T-cell responses, and protective immunity without vaccine-enhanced disease (VED) against RSV.
MATERIALS AND METHODS
We used RSV G protein fragment (Gcf A) with recombinant baculovirus capable of expressing the RSV M2 protein (Bac M2) as a vaccine candidate, and injected this vaccine (Gcf A/Bac M2) intramuscularly, and challenged with RSV intranasally into mice. Enzyme-linked immunosorbent assay, flow cytometry, plaque assay, and weight measurement were performed to confirm humoral immunity, cellular immunity, and protective immunity.
RESULTS
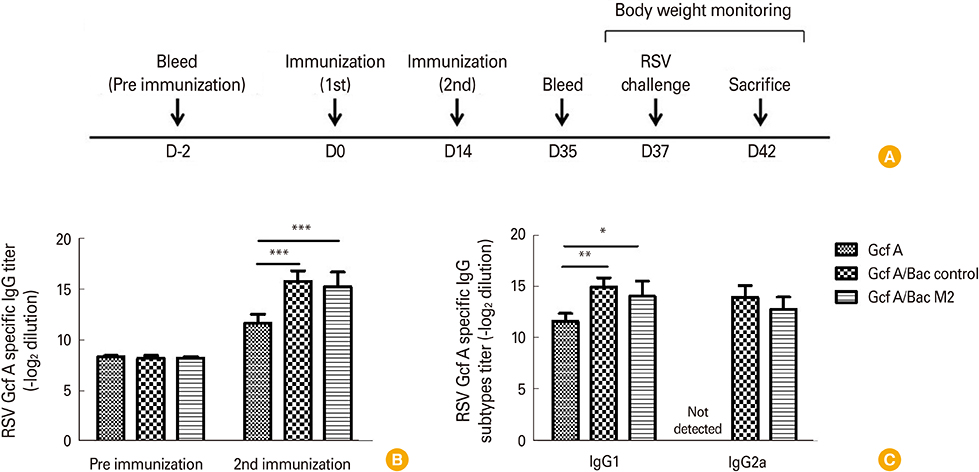
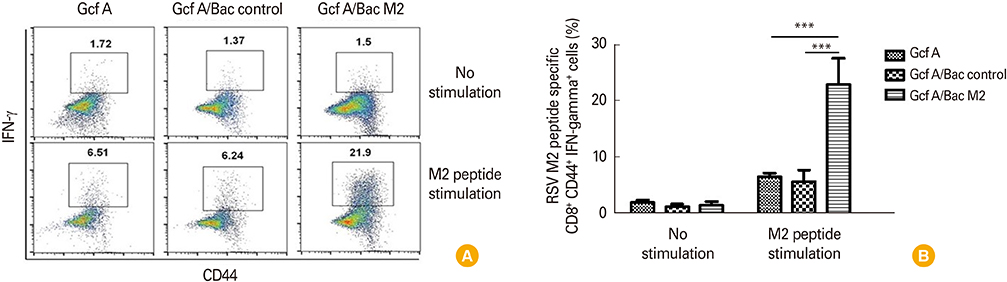
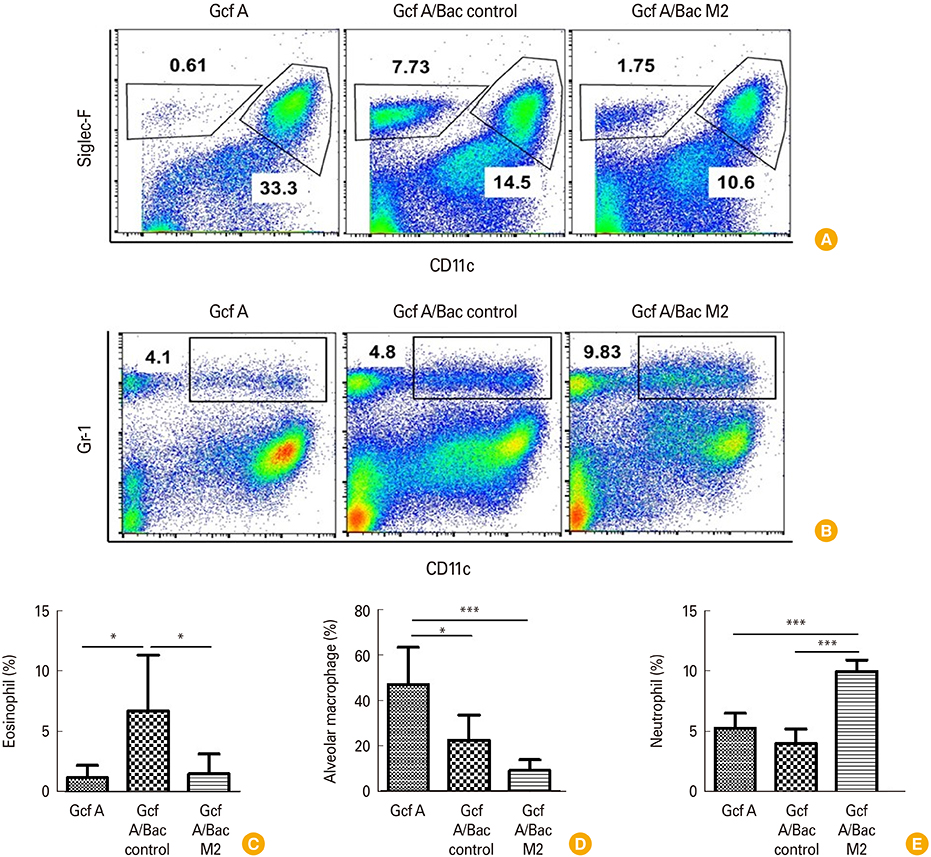
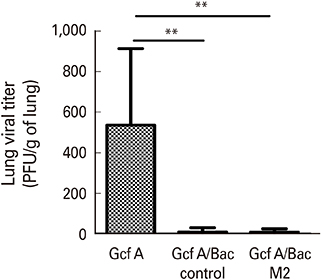
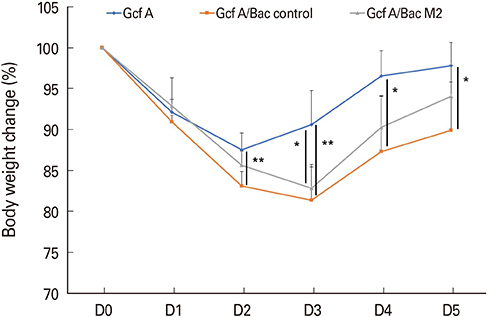
The Gcf A/Bac M2 formulation induced a stronger IgG response to Gcf A than Gcf A inoculation alone, and the ratio of IgG1/IgG2a indicated that the responses shifted predominantly to Th1. In addition, both RSV G-specific Th1 responses and RSV M2-specific CD8+ T-cell responses were induced, and G protein-associated eosinophilic infiltration was suppressed compared to the control group. Moreover, the Gcf A/Bac M2 group showed effective protection after an RSV challenge.
CONCLUSION
Bac M2 could serve as a vaccine with intrinsic adjuvant activity, and the Gcf A/Bac M2 shows promise as a vaccine candidate for inducing protective immunity without inciting VED.
MeSH Terms
Figure
Reference
-
1. Lee JY, Chang J. Universal vaccine against respiratory syncytial virus A and B subtypes. PLoS One. 2017; 12:e0175384.
Article2. Jeong KI, Piepenhagen PA, Kishko M, et al. CX3CR1 is expressed in differentiated human ciliated airway cells and co-localizes with respiratory syncytial virus on cilia in a G protein-dependent manner. PLoS One. 2015; 10:e0130517.
Article3. Schmidt ME, Varga SM. Modulation of the host immune response by respiratory syncytial virus proteins. J Microbiol. 2017; 55:161–171.
Article4. Openshaw PJ, Chiu C, Culley FJ, Johansson C. Protective and harmful immunity to RSV infection. Annu Rev Immunol. 2017; 35:501–532.
Article5. Citron MP, Patel M, Purcell M, et al. A novel method for strict intranasal delivery of non-replicating RSV vaccines in cotton rats and non-human primates. Vaccine. 2018; 36:2876–2885.
Article6. Killikelly AM, Kanekiyo M, Graham BS. Pre-fusion F is absent on the surface of formalin-inactivated respiratory syncytial virus. Sci Rep. 2016; 6:34108.
Article7. Boyoglu-Barnum S, Todd SO, Meng J, et al. Mutating the CX3C motif in the G protein should make a live respiratory syncytial virus vaccine safer and more effective. J Virol. 2017; 91:e02059-16.
Article8. Cheon IS, Shim BS, Park SM, et al. Development of safe and effective RSV vaccine by modified CD4 epitope in G protein core fragment (Gcf). PLoS One. 2014; 9:e94269.
Article9. Kim S, Joo DH, Lee JB, et al. Dual role of respiratory syncytial virus glycoprotein fragment as a mucosal immunogen and chemotactic adjuvant. PLoS One. 2012; 7:e32226.
Article10. Lee JY, Chang J. Recombinant baculovirus-based vaccine expressing M2 protein induces protective CD8(+) T-cell immunity against respiratory syncytial virus infection. J Microbiol. 2017; 55:900–908.
Article11. Bar-Haim E, Erez N, Malloy AM, Graham BS, Ruckwardt TJ. CD8+ TCR transgenic strains expressing public versus private TCR targeting the respiratory syncytial virus K(d)M2(82–90) epitope demonstrate similar functional profiles. PLoS One. 2014; 9:e99249.
Article12. Kulkarni AB, Morse HC 3rd, Bennink JR, Yewdell JW, Murphy BR. Immunization of mice with vaccinia virus-M2 recombinant induces epitope-specific and cross-reactive Kd-restricted CD8+ cytotoxic T cells. J Virol. 1993; 67:4086–4092.
Article13. Kim S, Chang J. Baculovirus-based vccine dsplaying rspiratory sncytial vrus glycoprotein iduces potective imunity against RSV ifection without vccine-ehanced dsease. Immune Netw. 2012; 12:8–17.
Article14. Varga SM, Welsh RM. High frequency of virus-specific interleukin-2-producing CD4(+) T cells and Th1 dominance during lymphocytic choriomeningitis virus infection. J Virol. 2000; 74:4429–4432.
Article15. Li C, Zhou X, Zhong Y, et al. A recombinant G protein plus cyclosporine A-based respiratory syncytial virus vaccine elicits humoral and regulatory T cell responses against infection without vaccine-enhanced disease. J Immunol. 2016; 196:1721–1731.
Article16. Hua Y, Jiao YY, Ma Y, et al. DNA vaccine encoding central conserved region of G protein induces Th1 predominant immune response and protection from RSV infection in mice. Immunol Lett. 2016; 179:95–101.
Article17. Castilow EM, Varga SM. Overcoming T cell-mediated immunopathology to achieve safe RSV vaccination. Future Virol. 2008; 3:445–454.
Article18. Blanco JC, Boukhvalova MS, Shirey KA, Prince GA, Vogel SN. New insights for development of a safe and protective RSV vaccine. Hum Vaccin. 2010; 6:482–492.
Article19. Connors M, Giese NA, Kulkarni AB, Firestone CY, Morse HC 3rd, Murphy BR. Enhanced pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of interleukin-4 (IL-4) and IL-10. J Virol. 1994; 68:5321–5325.
Article20. Durant LR, Makris S, Voorburg CM, Loebbermann J, Johansson C, Openshaw PJ. Regulatory T cells prevent Th2 immune responses and pulmonary eosinophilia during respiratory syncytial virus infection in mice. J Virol. 2013; 87:10946–10954.
Article21. Graham BS. Vaccines against respiratory syncytial virus: the time has finally come. Vaccine. 2016; 34:3535–3541.
Article22. Mazur NI, Higgins D, Nunes MC, et al. The respiratory syncytial virus vaccine landscape: lessons from the graveyard and promising candidates. Lancet Infect Dis. 2018; 18:e295–e311.
Article23. Boyce FM, Bucher NL. Baculovirus-mediated gene transfer into mammalian cells. Proc Natl Acad Sci U S A. 1996; 93:2348–2352.
Article24. Hervas-Stubbs S, Rueda P, Lopez L, Leclerc C. Insect baculoviruses strongly potentiate adaptive immune responses by inducing type I IFN. J Immunol. 2007; 178:2361–2369.
Article25. Bielefeldt-Ohmann H, Beasley DW, Fitzpatrick DR, Aaskov JG. Analysis of a recombinant dengue-2 virus-dengue-3 virus hybrid envelope protein expressed in a secretory baculovirus system. J Gen Virol. 1997; 78(Pt 11):2723–2733.
Article26. Ruitenberg KM, Walker C, Love DN, Wellington JE, Whalley JM. A prime-boost immunization strategy with DNA and recombinant baculovirus-expressed protein enhances protective immunogenicity of glycoprotein D of equine herpesvirus 1 in naive and infection-primed mice. Vaccine. 2000; 18:1367–1373.
Article27. Chen CY, Lin SY, Cheng MC, et al. Baculovirus vector as an avian influenza vaccine: hemagglutinin expression and presentation augment the vaccine immunogenicity. J Biotechnol. 2013; 164:143–150.
Article28. Grabowska AK, Lipinska AD, Rohde J, Szewczyk B, Bienkowska-Szewczyk K, Rziha HJ. New baculovirus recombinants expressing Pseudorabies virus (PRV) glycoproteins protect mice against lethal challenge infection. Vaccine. 2009; 27:3584–3591.
Article29. Heinimaki S, Tamminen K, Malm M, Vesikari T, Blazevic V. Live baculovirus acts as a strong B and T cell adjuvant for monomeric and oligomeric protein antigens. Virology. 2017; 511:114–122.
Article30. Hsu SC, Chargelegue D, Steward MW. Reduction of respiratory syncytial virus titer in the lungs of mice after intranasal immunization with a chimeric peptide consisting of a single CTL epitope linked to a fusion peptide. Virology. 1998; 240:376–381.
Article31. Chang J. Current progress on development of respiratory syncytial virus vaccine. BMB Rep. 2011; 44:232–237.
Article32. Nicholas JA, Rubino KL, Levely ME, Adams EG, Collins PL. Cytolytic T-lymphocyte responses to respiratory syncytial virus: effector cell phenotype and target proteins. J Virol. 1990; 64:4232–4241.
Article33. Openshaw PJ, Culley FJ, Olszewska W. Immunopathogenesis of vaccine-enhanced RSV disease. Vaccine. 2001; 20:Suppl 1. S27–S31.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Baculovirus-based Vaccine Displaying Respiratory Syncytial Virus Glycoprotein Induces Protective Immunity against RSV Infection without Vaccine-Enhanced Disease
- In Hot Pursuit of the First Vaccine Against Respiratory Syncytial Virus
- Immunogenicity and Protective Efficacy of a Dual Subunit Vaccine Against Respiratory Syncytial Virus and Influenza Virus
- In vivo CTL Activity Induced by Prime-boost Vaccination using Recombinant Vaccinia Virus and DNA Vaccine Expressing Epitope Specific for CD8+ T Cells
- Construction of a Novel Recombinant Baculovirus Producing Polyhedra with Bacillus thuringiensis Cry1Ac Crystal Protein