Clin Exp Vaccine Res.
2019 Jan;8(1):27-34. 10.7774/cevr.2019.8.1.27.
Expression and serological application of recombinant epitope-repeat protein carrying an immunodominant epitope of Newcastle disease virus nucleoprotein
- Affiliations
-
- 1Department of Veterinary Microbiology, College of Veterinary and Animal Sciences, Parbhani, India.
- 2Avian Disease Research Division, Animal and Plant Quarantine Agency, Gimcheon, Korea.
- 3Veterinary Drugs and Biologics Division, Animal and Plant Quarantine Agency, Gimcheon, Korea.
- 4Planning and Coordination Division, Animal and Plant Quarantine Agency, Gimcheon, Korea. kchoi0608@korea.kr
- KMID: 2438958
- DOI: http://doi.org/10.7774/cevr.2019.8.1.27
Abstract
- PURPOSE
The aim of the present study was to develop a serodiagnostic test for differentiation infected from vaccinated animal (DIVA) strategy accompanying the marker vaccine lacking an immunodominant epitope (IDE) of nucleoprotein of Newcastle disease virus (NDV).
MATERIALS AND METHODS
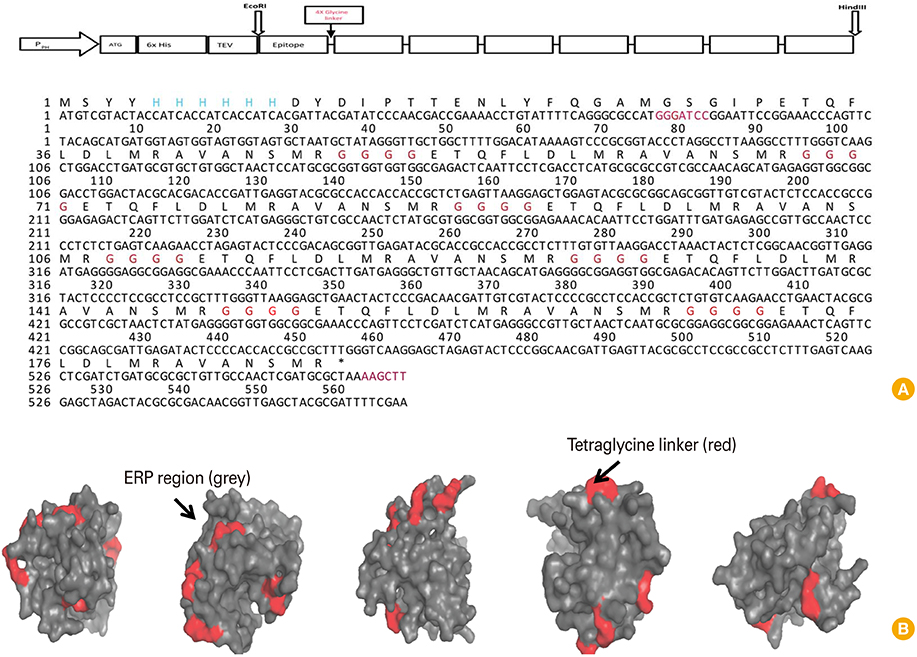
Recombinant epitope-repeat protein (rERP) gene encoding eight repeats of the IDE sequence (ETQFLDLMRAVANSMR) by tetra-glycine linker was synthesized. Recombinant baculovirus carrying the rERP gene was generated to express the rERP in insect cells. Specificity and sensitivity of an indirect enzyme-linked immunosorbent assay (ELISA) employing the rERP was evaluated.
RESULTS
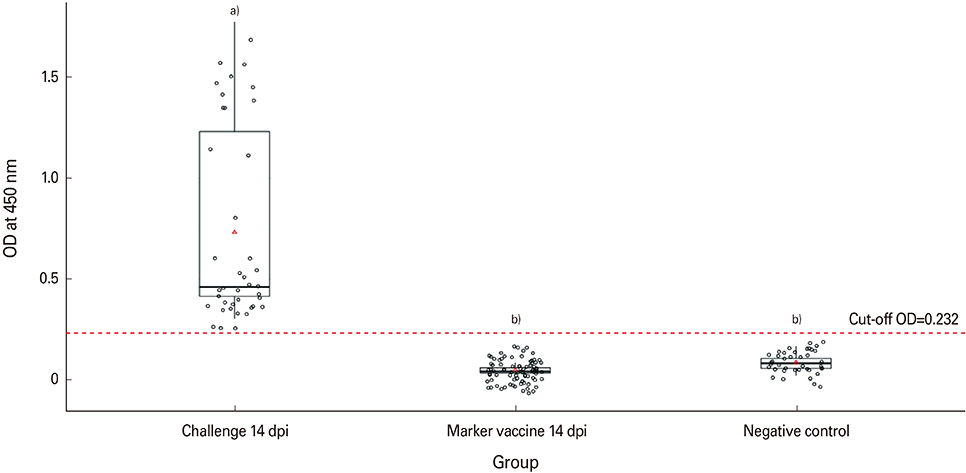
The rERP with molecular weight of 20 kDa was successfully expressed by the recombinant baculovirus in an insect-baculovirus system. The rERP was antigenically functional as demonstrated by Western blotting. An indirect ELISA employing the rERP was developed and its specificity and sensitivity was determined. The ELISA test allowed discrimination of NDV infected sera from epitope deletion virus vaccinated sera.
CONCLUSION
The preliminary results represent rERP ELISA as a promising DIVA diagnostic tool.
Keyword
MeSH Terms
Figure
Reference
-
1. Miller PJ, Torchetti MK. Newcastle disease virus detection and differentiation from avian influenza. Methods Mol Biol. 2014; 1161:235–239.
Article2. Diel DG, da Silva LH, Liu H, Wang Z, Miller PJ, Afonso CL. Genetic diversity of avian paramyxovirus type 1: proposal for a unified nomenclature and classification system of Newcastle disease virus genotypes. Infect Genet Evol. 2012; 12:1770–1779.
Article3. Mohan CM, Dey S, Rai A, Kataria JM. Recombinant haemagglutinin neuraminidase antigen-based single serum dilution ELISA for rapid serological profiling of Newcastle disease virus. J Virol Methods. 2006; 138:117–122.
Article4. Mebatsion T, Koolen MJ, de Vaan LT, et al. Newcastle disease virus (NDV) marker vaccine: an immunodominant epitope on the nucleoprotein gene of NDV can be deleted or replaced by a foreign epitope. J Virol. 2002; 76:10138–10146.
Article5. Ahmad-Raus R, Ali AM, Tan WS, Salleh HM, Eshaghi M, Yusoff K. Localization of the antigenic sites of Newcastle disease virus nucleocapsid using a panel of monoclonal antibodies. Res Vet Sci. 2009; 86:174–182.
Article6. Uttenthal A, Parida S, Rasmussen TB, Paton DJ, Haas B, Dundon WG. Strategies for differentiating infection in vaccinated animals (DIVA) for foot-and-mouth disease, classical swine fever and avian influenza. Expert Rev Vaccines. 2010; 9:73–87.
Article7. AnandaRao R, Swaminathan S, Fernando S, Jana AM, Khanna N. A custom-designed recombinant multiepitope protein as a dengue diagnostic reagent. Protein Expr Purif. 2005; 41:136–147.
Article8. Huang XJ, Lu X, Lei YF, et al. Cellular immunogenicity of a multi-epitope peptide vaccine candidate based on hepatitis C virus NS5A, NS4B and core proteins in HHD-2 mice. J Virol Methods. 2013; 189:47–52.
Article9. Oany AR, Emran AA, Jyoti TP. Design of an epitope-based peptide vaccine against spike protein of human coronavirus: an in silico approach. Drug Des Devel Ther. 2014; 8:1139–1149.
Article10. Spearman P, Kalams S, Elizaga M, et al. Safety and immunogenicity of a CTL multiepitope peptide vaccine for HIV with or without GM-CSF in a phase I trial. Vaccine. 2009; 27:243–249.
Article11. Cho HI, Celis E. Design of immunogenic and effective multi-epitope DNA vaccines for melanoma. Cancer Immunol Immunother. 2012; 61:343–351.
Article12. Tian L, Wang HN, Lu D, Zhang YF, Wang T, Kang RM. The immunoreactivity of a chimeric multi-epitope DNA vaccine against IBV in chickens. Biochem Biophys Res Commun. 2008; 377:221–225.
Article13. de Souza MQ, Galdino AS, dos Santos JC, et al. A recombinant multiepitope protein for hepatitis B diagnosis. Biomed Res Int. 2013; 2013:148317.
Article14. Bhatnagar S, Kumar P, Mohan T, et al. Evaluation of multiple antigenic peptides based on the Chikungunya E2 protein for improved serological diagnosis of infection. Viral Immunol. 2015; 28:107–112.
Article15. Cheng Z, Zhao JW, Sun ZQ, et al. Evaluation of a novel fusion protein antigen for rapid serodiagnosis of tuberculosis. J Clin Lab Anal. 2011; 25:344–349.
Article16. Lin X, Chen Y, Yan J. Recombinant multiepitope protein for diagnosis of leptospirosis. Clin Vaccine Immunol. 2008; 15:1711–1714.
Article17. Zhang Y. I-TASSER: fully automated protein structure prediction in CASP8. Proteins. 2009; 77:Suppl 9. 100–113.
Article18. DeLano WL. The PyMOL molecular graphics system. Palo Alto: DeLano Scientific;2002.19. Jeon WJ, Lee EK, Lee YJ, et al. Protective efficacy of commercial inactivated Newcastle disease virus vaccines in chickens against a recent Korean epizootic strain. J Vet Sci. 2008; 9:295–300.
Article20. Chander V, Nandi S, Ravishankar C, Upmanyu V, Verma R. Classical swine fever in pigs: recent developments and future perspectives. Anim Health Res Rev. 2014; 15:87–101.
Article21. Takada A, Kida H. Protective immune response of chickens against Newcastle disease, induced by the intranasal vaccination with inactivated virus. Vet Microbiol. 1996; 50:17–25.
Article22. Kamiya N, Niikura M, Ono M, Kai C, Matsuura Y, Mikami T. Protective effect of individual glycoproteins of Newcastle disease virus expressed in insect cells: the fusion protein derived from an avirulent strain had lower protective efficacy. Virus Res. 1994; 32:373–379.
Article23. Guan D, Chen Z. Challenges and recent advances in affinity purification of tag-free proteins. Biotechnol Lett. 2014; 36:1391–1406.
Article24. Kimple ME, Brill AL, Pasker RL. Overview of affinity tags for protein purification. Curr Protoc Protein Sci. 2013; 73:Unit 9.9.
Article25. Mauro VP, Chappell SA. A critical analysis of codon optimization in human therapeutics. Trends Mol Med. 2014; 20:604–613.
Article26. Tanaka M, Tokuoka M, Gomi K. Effects of codon optimization on the mRNA levels of heterologous genes in filamentous fungi. Appl Microbiol Biotechnol. 2014; 98:3859–3867.
Article27. Elena C, Ravasi P, Castelli ME, Peiru S, Menzella HG. Expression of codon optimized genes in microbial systems: current industrial applications and perspectives. Front Microbiol. 2014; 5:21.
Article28. Klement M, Liu C, Loo BL, Choo AB, Ow DS, Lee DY. Effect of linker flexibility and length on the functionality of a cytotoxic engineered antibody fragment. J Biotechnol. 2015; 199:90–97.
Article29. Villaflores OB, Hsei CM, Teng CY, et al. Easy expression of the C-terminal heavy chain domain of botulinum neurotoxin serotype A as a vaccine candidate using a bi-cistronic baculovirus system. J Virol Methods. 2013; 189:58–64.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antigenic and immunogenic investigation of the virulence motif of the Newcastle disease virus fusion protein
- Robust immunoreactivity of teenager sera against peptide 19 from Porphyromonas gingivalis HSP60
- Expression of F Protein Gene of a Thermostable Isolate of Newcastle Disease Virus Using Baculovirus Expression System
- Nucleoprotein vaccine induces cross-protective cytotoxic T lymphocytes against both lineages of influenza B virus
- Construction and Characterization of Recombinant Poliovirus that Delivers T-cell epitope