Cancer Res Treat.
2019 Jan;51(1):150-157. 10.4143/crt.2017.476.
The Prognostic Role of Circulating Epstein-Barr Virus DNA Copy Number in Angioimmunoblastic T-Cell Lymphoma Treated with Dose-Adjusted EPOCH
- Affiliations
-
- 1Department of Hematology, The First Affiliated Hospital of Nanjing Medical University, Jiangsu Province Hospital, Nanjing, China. xuwei10000@hotmail.com, lijianyonglm@medmail.com.cn
- 2Key Laboratory of Hematology of Nanjing Medical University, Nanjing, China.
- 3Collaborative Innovation Center for Cancer Personalized Medicine, Nanjing, China.
- KMID: 2437608
- DOI: http://doi.org/10.4143/crt.2017.476
Abstract
- PURPOSE
Determine the frequency and prognostic value of circulating Epstein-Barr virus (EBV) DNA copy number in angioimmunoblastic T-cell lymphoma (AITL) patients who were treated with dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide and doxorubicin (DA-EPOCH) regimens.
MATERIALS AND METHODS
Sixty newly-diagnosed AITL patients were retrospectively enrolled in the present study. All patients were treated with DA-EPOCH regimen.
RESULTS
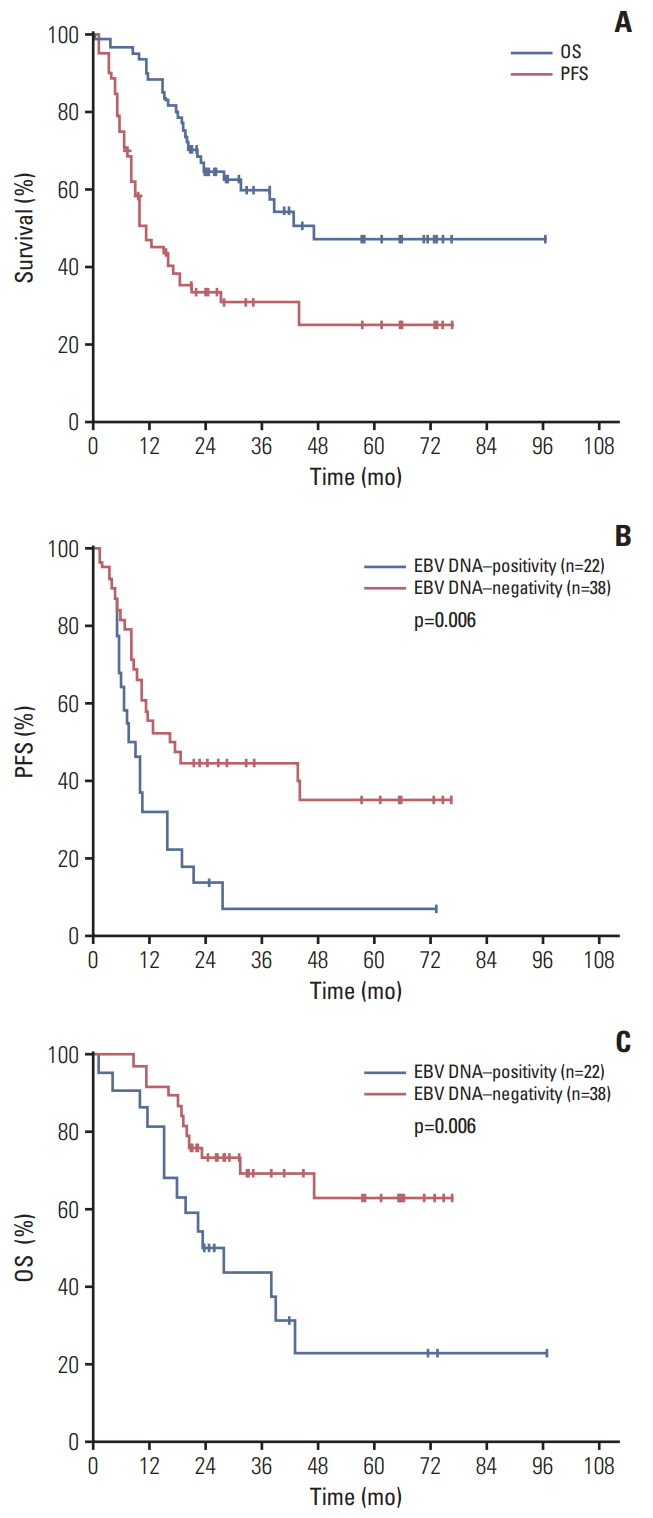
Twenty-two subjects (36.7%) had a EBV DNA-positive test at diagnosis. EBV DNA"’positive patients were associated with lower lymphocyte-monocyte ratio (p=0.024). Median follow-up was 40 months (range, 14 to 100 months). The overall response rate for all the 60 AITL patents were 71.7% (95% confidence interval [CI], 58.6 to 82.5) with 3-year progressive-free survival (PFS) rate of 30.9%±6.1% and overall survival (OS) rate of 60.1%±6.6%. Not only did PFS estimation differ between the EBV DNA"’positive and EBV DNA"’negative group (hazard ratio [HR], 2.24; 95% CI, 1.15 to 4.35; p=0.006), but also worse OS was observed in the pretreatment EBV DNA"’positive group than in the EBV DNA"’negative group (HR, 2.74; 95% CI, 1.22 to 6.19; p=0.006). EBV DNA test positivity was independent prognostic marker for both PFS (HR, 2.17; 95% CI, 1.17 to 4.00; p=0.014) and OS (HR, 3.24; 95% CI, 1.48 to 7.11; p=0.004) after adjusting International Prognostic Index and prognostic index for AITL score. Reduction in EBV copies was significantly associated with therapy-response.
CONCLUSION
Circulating EBV DNA level was an important prognostic and monitoring marker for AITL patients who treated with DA-EPOCH regimens which cannot improve outcomes for AITL patients.
MeSH Terms
Figure
Reference
-
References
1. Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO classication of tumours of haematopoietic and lymphoid tissues. Lyon: IARC Press;2008.2. Mourad N, Mounier N, Briere J, Raffoux E, Delmer A, Feller A, et al. Clinical, biologic, and pathologic features in 157 patients with angioimmunoblastic T-cell lymphoma treated within the Groupe d'Etude des Lymphomes de l'Adulte (GELA) trials. Blood. 2008; 111:4463–70.
Article3. Vinuesa CG, Tangye SG, Moser B, Mackay CR. Follicular B helper T cells in antibody responses and autoimmunity. Nat Rev Immunol. 2005; 5:853–65.
Article4. Zhou Y, Attygalle AD, Chuang SS, Diss T, Ye H, Liu H, et al. Angioimmunoblastic T-cell lymphoma: histological progression associates with EBV and HHV6B viral load. Br J Haematol. 2007; 138:44–53.
Article5. Huang J, Zhang PH, Gao YH, Qiu LG. Sequential development of diffuse large B-cell lymphoma in a patient with angioimmunoblastic T-cell lymphoma. Diagn Cytopathol. 2012; 40:346–51.
Article6. Hsu SM, Hsu PL. Autocrine and paracrine functions of cytokines in malignant lymphomas. Biomed Pharmacother. 1994; 48:433–44.
Article7. Ito Y, Kimura H, Maeda Y, Hashimoto C, Ishida F, Izutsu K, et al. Pretreatment EBV-DNA copy number is predictive of response and toxicities to SMILE chemotherapy for extranodal NK/T-cell lymphoma, nasal type. Clin Cancer Res. 2012; 18:4183–90.
Article8. Kim SJ, Yoon DH, Jaccard A, Chng WJ, Lim ST, Hong H, et al. A prognostic index for natural killer cell lymphoma after non-anthracycline-based treatment: a multicentre, retrospective analysis. Lancet Oncol. 2016; 17:389–400.
Article9. Liang JH, Gao R, Xia Y, Gale RP, Chen RZ, Yang YQ, et al. Prognostic impact of Epstein-Barr virus (EBV)-DNA copy number at diagnosis in chronic lymphocytic leukemia. Oncotarget. 2016; 7:2135–42.
Article10. Liang JH, Lu TX, Tian T, Wang L, Fan L, Xu J, et al. Epstein-Barr virus (EBV) DNA in whole blood as a superior prognostic and monitoring factor than EBV-encoded small RNA in situ hybridization in diffuse large B-cell lymphoma. Clin Microbiol Infect. 2015; 21:596–602.
Article11. Liang JH, Wang L, Peter Gale R, Wu W, Xia Y, Fan L, et al. Efficacy of pegaspargase, etoposide, methotrexate and dexamethasone in newly diagnosed advanced-stage extra-nodal natural killer/T-cell lymphoma with the analysis of the prognosis of whole blood EBV-DNA. Blood Cancer J. 2017; 7:e608.
Article12. Chen Y, Zheng X, Chen B, Yang X, Zheng J, Zheng Z, et al. The clinical significance of Epstein-Barr virus DNA in peripheral blood mononuclear cells in patients with non-Hodgkin lymphoma. Leuk Lymphoma. 2017; 58:2349–55.
Article13. Lai GM, Chen YN, Mickley LA, Fojo AT, Bates SE. P-glycoprotein expression and schedule dependence of adriamycin cytotoxicity in human colon carcinoma cell lines. Int J Cancer. 1991; 49:696–703.
Article14. Wilson WH, Grossbard ML, Pittaluga S, Cole D, Pearson D, Drbohlav N, et al. Dose-adjusted EPOCH chemotherapy for untreated large B-cell lymphomas: a pharmacodynamic approach with high efficacy. Blood. 2002; 99:2685–93.
Article15. Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999; 17:1244.16. Weiss LM, Jaffe ES, Liu XF, Chen YY, Shibata D, Medeiros LJ. Detection and localization of Epstein-Barr viral genomes in angioimmunoblastic lymphadenopathy and angioimmunoblastic lymphadenopathy-like lymphoma. Blood. 1992; 79:1789–95.
Article17. Delfau-Larue MH, de Leval L, Joly B, Plonquet A, Challine D, Parrens M, et al. Targeting intratumoral B cells with rituximab in addition to CHOP in angioimmunoblastic T-cell lymphoma: a clinicobiological study of the GELA. Haematologica. 2012; 97:1594–602.
Article18. Nickelsen M, Ziepert M, Zeynalova S, Glass B, Metzner B, Leithaeuser M, et al. High-dose CHOP plus etoposide (Mega-CHOEP) in T-cell lymphoma: a comparative analysis of patients treated within trials of the German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL). Ann Oncol. 2009; 20:1977–84.
Article19. Karakas T, Bergmann L, Stutte HJ, Jager E, Knuth A, Weidmann E, et al. Peripheral T-cell lymphomas respond well to vincristine, adriamycin, cyclophosphamide, prednisone and etoposide (VACPE) and have a similar outcome as high-grade B-cell lymphomas. Leuk Lymphoma. 1996; 24:121–9.
Article20. Ganjoo K, Hong F, Horning SJ, Gascoyne RD, Natkunam Y, Swinnen LJ, et al. Bevacizumab and cyclosphosphamide, doxorubicin, vincristine and prednisone in combination for patients with peripheral T-cell or natural killer cell neoplasms: an Eastern Cooperative Oncology Group study (E2404). Leuk Lymphoma. 2014; 55:768–72.
Article21. Advani RH, Hong F, Horning SJ, Kahl BS, Manola J, Swinnen LJ, et al. Cardiac toxicity associated with bevacizumab (Avastin) in combination with CHOP chemotherapy for peripheral T cell lymphoma in ECOG 2404 trial. Leuk Lymphoma. 2012; 53:718–20.
Article22. de Leval L, Gisselbrecht C, Gaulard P. Advances in the understanding and management of angioimmunoblastic T-cell lymphoma. Br J Haematol. 2010; 148:673–89.
Article23. International Non-Hodgkin's Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med. 1993; 329:987–94.24. Federico M, Rudiger T, Bellei M, Nathwani BN, Luminari S, Coiffier B, et al. Clinicopathologic characteristics of angioimmunoblastic T-cell lymphoma: analysis of the international peripheral T-cell lymphoma project. J Clin Oncol. 2013; 31:240–6.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Epstein-Barr Virus-positive Diffuse, Large B-cell Lymphoma after Angioimmunoblastic T-cell Lymphoma
- A Case of Epstein-Barr Virus-Positive Diffuse Large B-Cell Lymphoma Occurring in Thyroid Gland
- Two Cases of the Angioimmunoblastic Lymphadenopathy Type of Peripheral T - cell Lymphoma : Different Clinical Courses According to Positivity to Epstein-Barr Virus
- A Case of Cutaneous Epstein-Barr Virus-Associated Diffuse Large B-Cell Lymphoma in an Angioimmunoblastic T-Cell Lymphoma
- Epstein-Barr Virus Positive Follicular Lymphoma of Lymph Node