J Pathol Transl Med.
2019 Jan;53(1):31-39. 10.4132/jptm.2018.11.16.
Uterine Malignant Mixed Müllerian Tumors Following Treatment with Selective Estrogen Receptor Modulators in Patients with Breast Cancer: A Report of 13 Cases and Their Clinicopathologic Characteristics
- Affiliations
-
- 1Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. krkim@amc.seoul.kr
- KMID: 2437576
- DOI: http://doi.org/10.4132/jptm.2018.11.16
Abstract
- BACKGROUND
Breast cancer treatment with selective estrogen receptor modulators (SERMs) increases the incidence of uterine malignant mixed Müllerian tumors (uMMMTs). We examine clinicopathologic characteristics and prognosis of SERM-associated uMMMTs (S-uMMMTs) and discuss possible pathogenetic mechanisms.
METHODS
Among 28,104 patients with breast cancer, clinicopathologic features and incidence of uMMMT were compared between patients who underwent SERM treatment and those who did not. Of 92 uMMMT cases that occurred during the same period, incidence, dose, and duration of SERM treatment, as well as overall survival rate, were compared for patients with breast cancer who underwent SERM treatment and those who did not (S-uMMMT vs NS-uMMMT) and for patients without breast cancer (de novo-uMMMT). Histopathological findings and immunophenotypes for myogenin, desmin, p53, WT-1, estrogen receptor (ER) α, ERβ, progesterone receptor, and GATA-3 were compared between S-uMMMT and de novo-uMMMT.
RESULTS
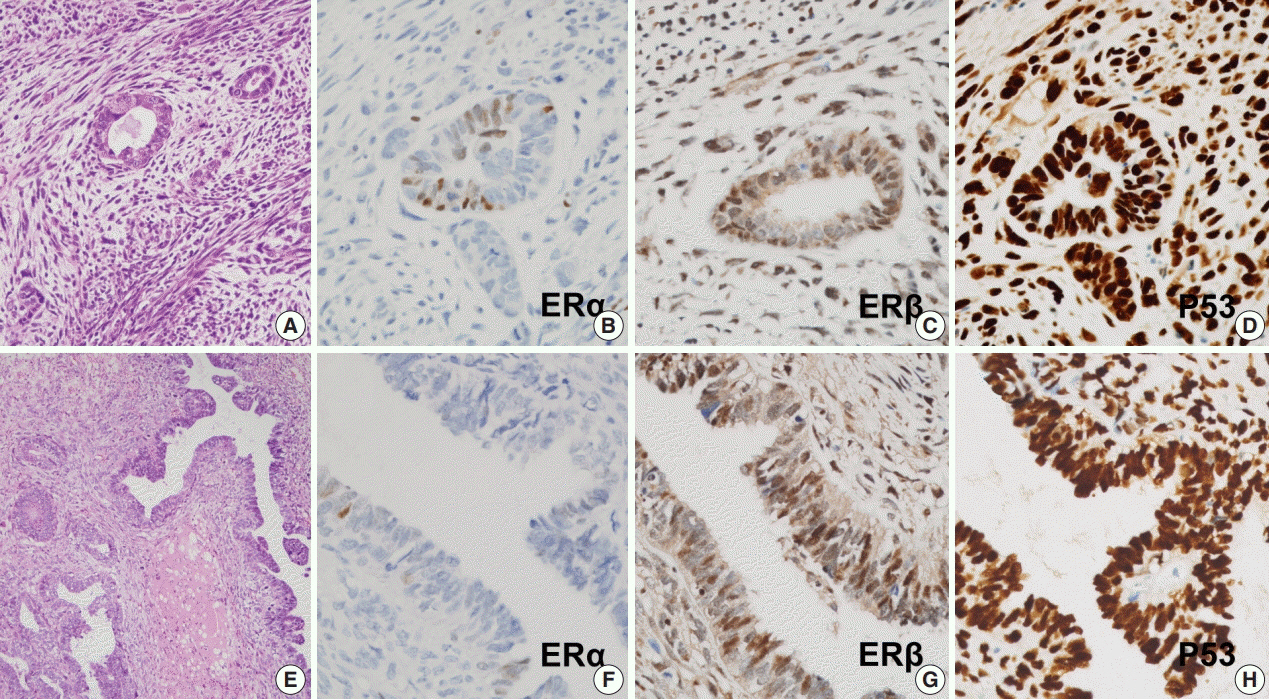
The incidence of S-uMMMT was significantly higher than that of NS-uMMMT (6.35-fold). All patients with SERM were postmenopausal and received daily 20-40 mg SERM. Cumulative SERM dose ranged from 21.9 to 73.0 g (mean, 46.0) over 39-192 months (mean, 107). Clinicopathologic features, such as International Federation of Gynecology and Obstetrics stage and overall survival, were not significantly different between patients with S-uMMMT and NS-uMMMT or between patients with S-uMMMT and de novo-uMMMT. All 11 S-uMMMT cases available for immunostaining exhibited strong overexpression/null expression of p53 protein and significantly increased ERβ expression in carcinomatous and sarcomatous components.
CONCLUSIONS
SERM therapy seemingly increases risk of S-uMMMT development; however, clinicopathologic features were similar in all uMMMTs from different backgrounds. p53 mutation and increased ERβ expression might be involved in the etiology of S-uMMMT.
Keyword
MeSH Terms
Figure
Reference
-
1. Kennedy BJ. Hormone therapy for advanced breast cancer. Cancer. 1965; 18:1551–7.
Article2. Elwood JM, Godolphin W. Oestrogen receptors in breast tumours: associations with age, menopausal status and epidemiological and clinical features in 735 patients. Br J Cancer. 1980; 42:635–44.
Article3. Cook LS, Weiss NS, Schwartz SM, et al. Population-based study of tamoxifen therapy and subsequent ovarian, endometrial, and breast cancers. J Natl Cancer Inst. 1995; 87:1359–64.
Article4. Webb P, Lopez GN, Uht RM, Kushner PJ. Tamoxifen activation of the estrogen receptor/AP-1 pathway: potential origin for the cellspecific estrogen-like effects of antiestrogens. Mol Endocrinol. 1995; 9:443–56.
Article5. Assikis VJ, Jordan VC. Gynecologic effects of tamoxifen and the association with endometrial carcinoma. Int J Gynaecol Obstet. 1995; 49:241–57.
Article6. van Leeuwen FE, Benraadt J, Coebergh JW, et al. Risk of endometrial cancer after tamoxifen treatment of breast cancer. Lancet. 1994; 343:448–52.
Article7. Fisher B, Costantino JP, Redmond CK, Fisher ER, Wickerham DL, Cronin WM. Endometrial cancer in tamoxifen-treated breast cancer patients: findings from the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14. J Natl Cancer Inst. 1994; 86:527–37.8. Barakat RR. The effect of tamoxifen on the endometrium. Oncology (Williston Park). 1995; 9:129–34.9. Bernstein L, Deapen D, Cerhan JR, et al. Tamoxifen therapy for breast cancer and endometrial cancer risk. J Natl Cancer Inst. 1999; 91:1654–62.
Article10. Bergman L, Beelen ML, Gallee MP, Hollema H, Benraadt J, van Leeuwen FE. Risk and prognosis of endometrial cancer after tamoxifen for breast cancer. Comprehensive Cancer Centres’ ALERT Group. Assessment of liver and endometrial cancer risk following tamoxifen. Lancet. 2000; 356:881–7.11. Jones ME, van Leeuwen FE, Hoogendoorn WE, et al. Endometrial cancer survival after breast cancer in relation to tamoxifen treatment: pooled results from three countries. Breast Cancer Res. 2012; 14:R91.
Article12. Wysowski DK, Honig SF, Beitz J. Uterine sarcoma associated with tamoxifen use. N Engl J Med. 2002; 346:1832–3.
Article13. Wickerham DL, Fisher B, Wolmark N, et al. Association of tamoxifen and uterine sarcoma. J Clin Oncol. 2002; 20:2758–60.
Article14. Lavie O, Barnett-Griness O, Narod SA, Rennert G. The risk of developing uterine sarcoma after tamoxifen use. Int J Gynecol Cancer. 2008; 18:352–6.
Article15. Eddy GL, Mazur MT. Endolymphatic stromal myosis associated with tamoxifen use. Gynecol Oncol. 1997; 64:262–4.
Article16. Christie DB 3rd, Day JD, Moore AB, Chapman JR, Nakayama DK, Conforti AM. Endometrial stromal sarcoma development after hysterectomy and tamoxifen therapy. Am Surg. 2008; 74:726–8.17. McCluggage WG, Abdulkader M, Price JH, et al. Uterine carcinosarcomas in patients receiving tamoxifen: a report of 19 cases. Int J Gynecol Cancer. 2000; 10:280–4.
Article18. Curtis RE, Freedman DM, Sherman ME, Fraumeni JF Jr. Risk of malignant mixed mullerian tumors after tamoxifen therapy for breast cancer. J Natl Cancer Inst. 2004; 96:70–4.
Article19. Cohen I, Altaras MM, Lew S, Tepper R, Beyth Y, Ben-Baruch G. Ovarian endometrioid carcinoma and endometriosis developing in a postmenopausal breast cancer patient during tamoxifen therapy: a case report and review of the literature. Gynecol Oncol. 1994; 55(3 Pt 1):443–7.
Article20. Yin L, Li J, Wei Y, Ma D, Sun Y, Sun Y. Primary ovarian small cell carcinoma of pulmonary type with coexisting endometrial carcinoma in a breast cancer patient receiving tamoxifen: a case report and literature review. Medicine (Baltimore). 2018; 97:e10900.21. Shushan A, Peretz T, Uziely B, Lewin A, Mor-Yosef S. Ovarian cysts in premenopausal and postmenopausal tamoxifen-treated women with breast cancer. Am J Obstet Gynecol. 1996; 174(1 Pt 1):141–4.
Article22. Cohen I, Figer A, Tepper R, et al. Ovarian overstimulation and cystic formation in premenopausal tamoxifen exposure: comparison between tamoxifen-treated and nontreated breast cancer patients. Gynecol Oncol. 1999; 72:202–7.
Article23. Kojima N, Yamasaki Y, Koh H, Miyashita M, Morita H. Long-acting luteinizing hormone-releasing hormone agonist for ovarian hyperstimulation induced by tamoxifen for breast cancer. Case Rep Obstet Gynecol. 2018; 2018:4931852.
Article24. Committee Opinion No. 601: Tamoxifen and uterine cancer. Obstet Gynecol. 2014; 123:1394–7.25. Pandey V, Zhang M, Chong QY, et al. Hypomethylation associated enhanced transcription of trefoil factor-3 mediates tamoxifen-stimulated oncogenicity of ER+ endometrial carcinoma cells. Oncotarget. 2017; 8:77268–91.
Article26. Wilson BT, Cordell HJ. Uterine carcinosarcoma/malignant mixed Mullerian tumor incidence is increased in women with breast cancer, but independent of hormone therapy. J Gynecol Oncol. 2015; 26:249–51.27. Biron-Shental T, Drucker L, Altaras M, Bernheim J, Fishman A. High incidence of BRCA1-2 germline mutations, previous breast cancer and familial cancer history in Jewish patients with uterine serous papillary carcinoma. Eur J Surg Oncol. 2006; 32:1097–100.
Article28. Pennington KP, Walsh T, Lee M, et al. BRCA1, TP53, and CHEK2 germline mutations in uterine serous carcinoma. Cancer. 2013; 119:332–8.29. Yemelyanova A, Vang R, Kshirsagar M, et al. Immunohistochemical staining patterns of p53 can serve as a surrogate marker for TP53 mutations in ovarian carcinoma: an immunohistochemical and nucleotide sequencing analysis. Mod Pathol. 2011; 24:1248–53.
Article30. Martinez ME, Wertheim BC, Natarajan L, et al. Reproductive factors, heterogeneity, and breast tumor subtypes in women of mexican descent. Cancer Epidemiol Biomarkers Prev. 2013; 22:1853–61.
Article31. Song N, Choi JY, Sung H, et al. Heterogeneity of epidemiological factors by breast tumor subtypes in Korean women: a case-case study. Int J Cancer. 2014; 135:669–81.
Article32. Ismail SM. Pathology of endometrium treated with tamoxifen. J Clin Pathol. 1994; 47:827–33.
Article33. Swerdlow AJ, Jones ME; For the British Tamoxifen Second Cancer Study Group. Tamoxifen treatment for breast cancer and risk of endometrial cancer: a case-control study. J Natl Cancer Inst. 2005; 97:375–84.
Article34. Vasconcelos AL, Nunes B, Duarte C, et al. Tamoxifen in breast cancer ipse dixit in uterine malignant mixed Mullerian tumor and sarcoma: a report of 8 cases and review of the literature. Rep Pract Oncol Radiother. 2013; 18:251–60.35. McInnes KJ, Andersson TC, Simonyte K, et al. Association of 11betahydroxysteroid dehydrogenase type I expression and activity with estrogen receptor beta in adipose tissue from postmenopausal women. Menopause. 2012; 19:1347–52.36. Thomas C, Gustafsson JA. A CUE hints at tumor resistance. Nat Med. 2011; 17:658–60.
Article37. Bado I, Nikolos F, Rajapaksa G, Gustafsson JÅ, Thomas C. ERbeta decreases the invasiveness of triple-negative breast cancer cells by regulating mutant p53 oncogenic function. Oncotarget. 2016; 7:13599–611.