Clin Exp Otorhinolaryngol.
2019 Feb;12(1):18-26. 10.21053/ceo.2017.01669.
Cochlear Damage Caused by the Striking Noise of Titanium Head Golf Driver
- Affiliations
-
- 1Department of Otolaryngology-Head and Neck Surgery, Brain Research Institute, College of Medicine, Chungnam National University, Daejeon, Korea.
- 2Department of Otolaryngology-Head and Neck Surgery, Wonkwang University College of Medicine, Iksan, Korea.
- 3Department of Otorhinolaryngology-Head and Neck Surgery, Dankook University College of Medicine, Cheonan, Korea.
- 4Department of Otorhinolaryngology-Head and Neck Surgery, SMG-SNU Boramae Medical Center, Seoul, Korea. yhkiment@gmail.com
- KMID: 2437487
- DOI: http://doi.org/10.21053/ceo.2017.01669
Abstract
OBJECTIVES
.: To investigate how mouse cochleae are affected by the striking noise of titanium head golf driver.
METHODS
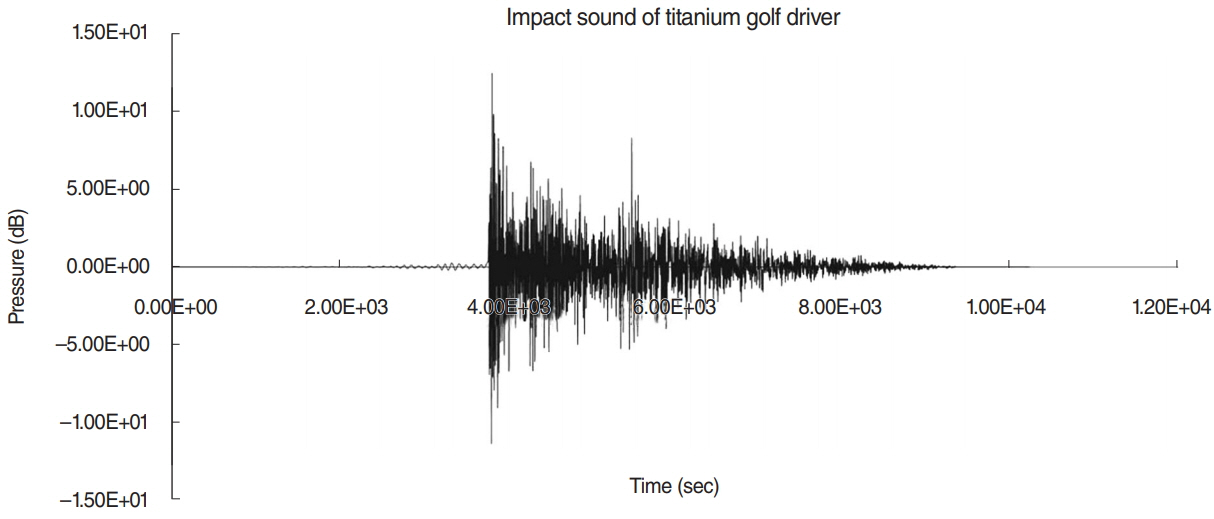
.: Thirty-two BALB/c mice (20-22 g) with normal hearing were used. The impact acoustic stimulus generated by the striking of titanium golf driver head was centered around 4.5 kHz with 120.5 dB sound pressure level. The recorded impact noise was provided to mice in two ways with the same exposure time of 288 seconds: 1,440 repetitions and an impact duration of 0.2 seconds for 2 hours (repetitive noise) or serially connected impact noise for 288 seconds (continuous noise). Auditory brainstem responses were measured at baseline, day 7, and day 14 after exposure. The mice were then sacrificed for histology.
RESULTS
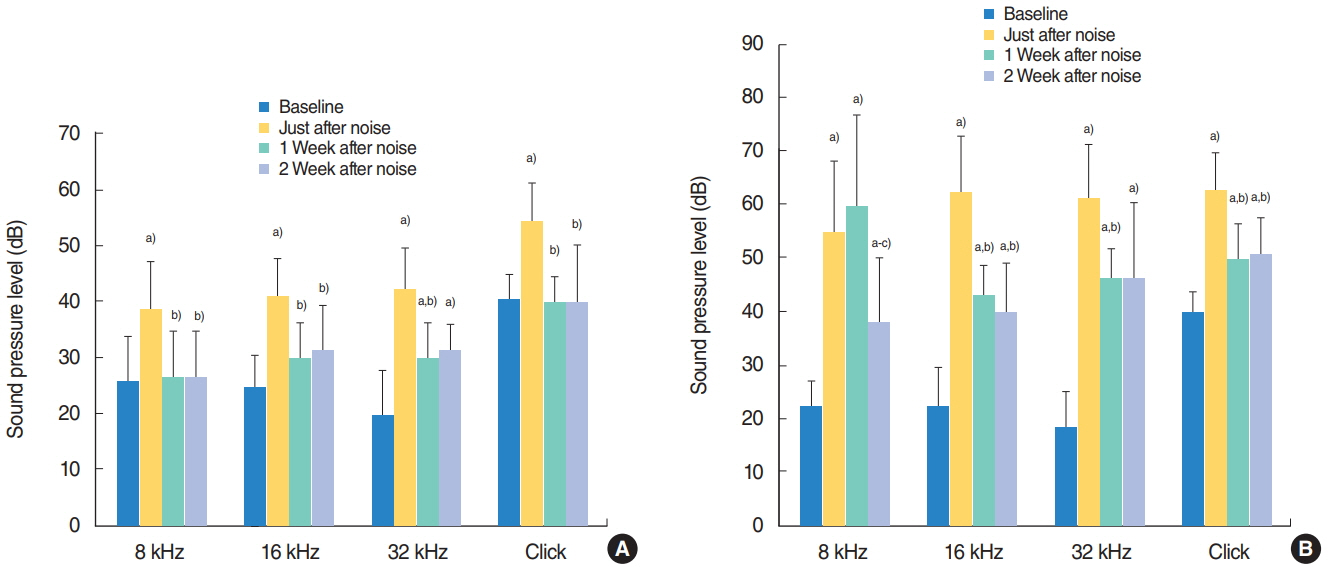
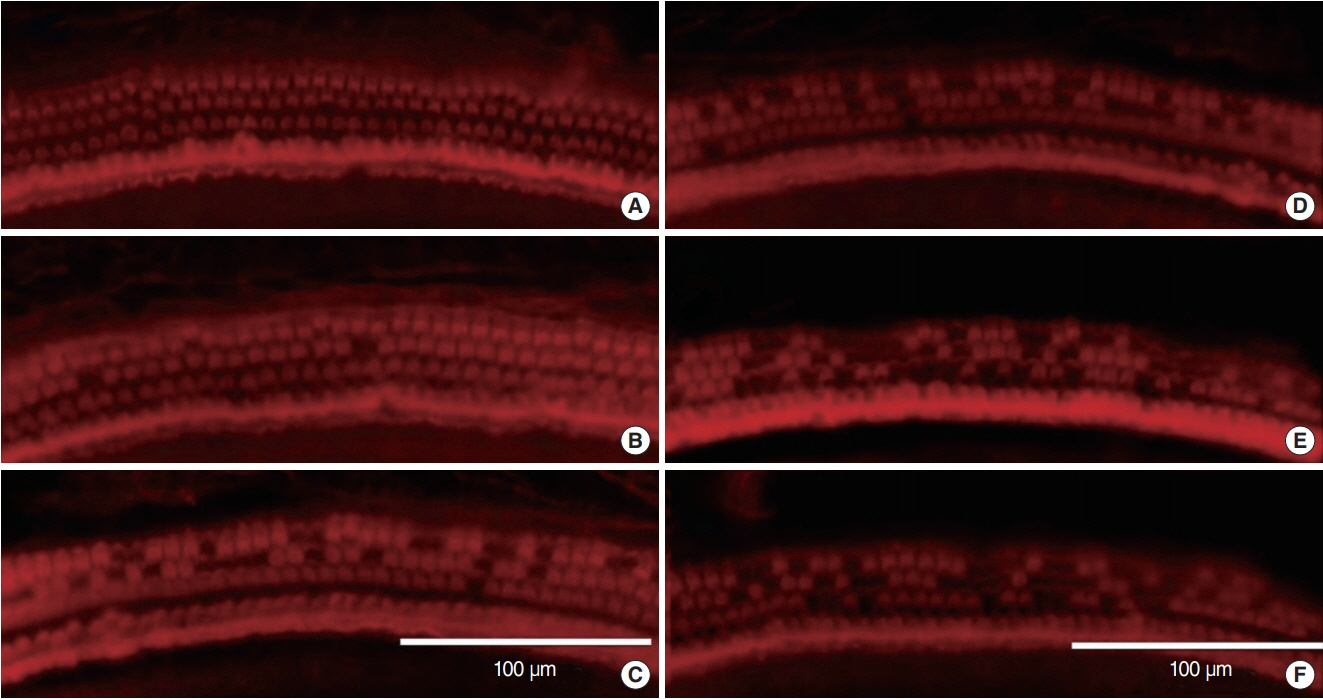
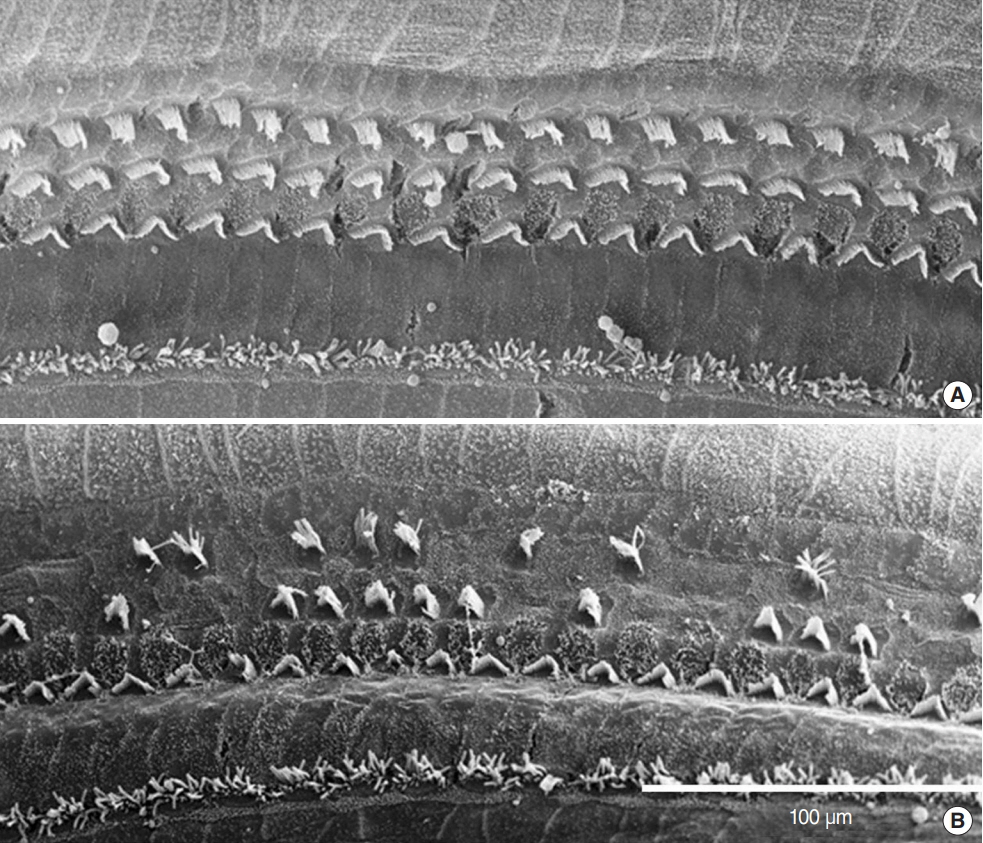
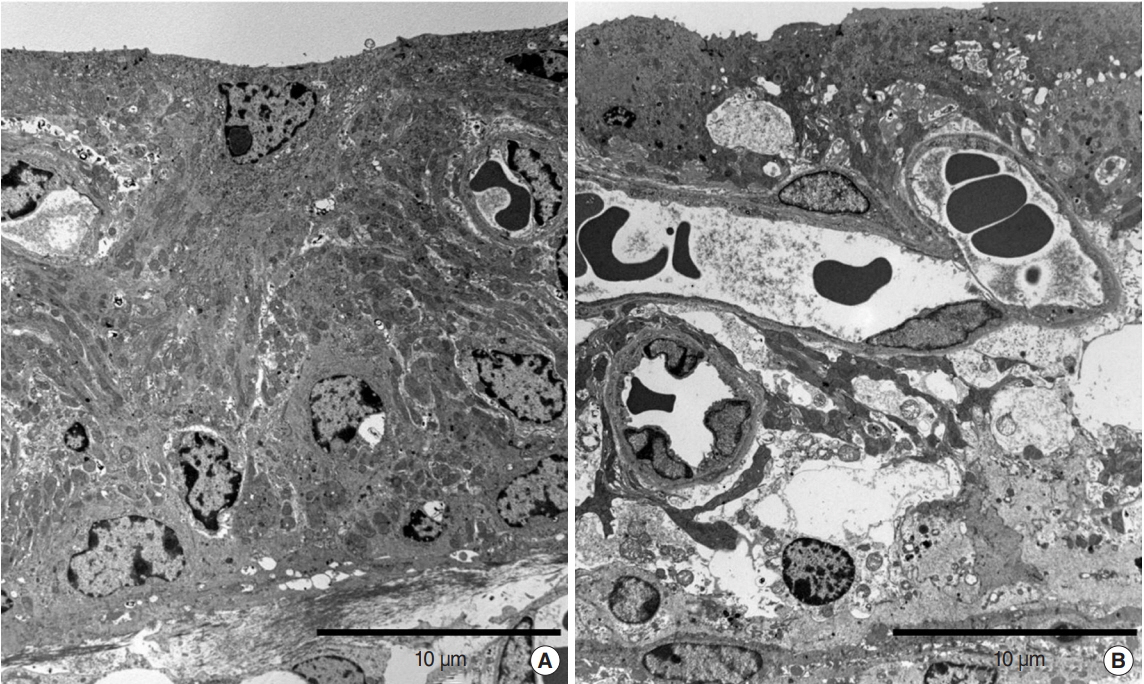
.: Both groups showed statistically significant threshold shifts immediately after noise exposure. Mice in the continuous exposure group, except for those exposed to 32 kHz noise, recovered from threshold shifts 1-2 weeks after noise exposure. However, in the repetitive exposure group, threshold shifts remained for 2 weeks after exposure. The repetitive exposure group had greater hair cell damage than did the continuous exposure group. Structural changes in the stria vascularis were observed in the repetitive exposure group.
CONCLUSION
.: Overexposure to impact noise caused by hitting of titanium head golf driver may be hazardous to the cochlea, and repetitive exposure may induce greater damage than continuous exposure.
Keyword
MeSH Terms
Figure
Reference
-
1. Buchanan MA, Wilkinson JM, Fitzgerald JE, Prinsley PR. Is golf bad for your hearing. BMJ. 2008; Dec. 337:a2835.
Article2. Zhao F, Bardsley B. Real-ear acoustical characteristics of impulse sound generated by golf drivers and the estimated risk to hearing: a cross-sectional study. BMJ Open. 2014; Jan. 4(1):e003517.
Article3. Kim YH, Kim YC, Lee JH, An YH, Park KT, Kang KM, et al. Analysis of impact noise induced by hitting of titanium head golf driver. Eur Arch Otorhinolaryngol. 2014; Nov. 271(11):2885–90.
Article4. Adelman C, Weinberger JM, Kriksunov L, Sohmer H. Effects of furosemide on the hearing loss induced by impulse noise. J Occup Med Toxicol. 2011; May. 6(1):14.
Article5. Bielefeld EC. Reduction in impulse noise-induced permanent threshold shift with intracochlear application of an NADPH oxidase inhibitor. J Am Acad Audiol. 2013; Jun. 24(6):461–73.
Article6. Kopke RD, Jackson RL, Coleman JK, Liu J, Bielefeld EC, Balough BJ. NAC for noise: from the bench top to the clinic. Hear Res. 2007; Apr. 226(1-2):114–25.
Article7. Le Prell CG, Hughes LF, Miller JM. Free radical scavengers vitamins A, C, and E plus magnesium reduce noise trauma. Free Radic Biol Med. 2007; May. 42(9):1454–63.8. Yamashita D, Jiang HY, Le Prell CG, Schacht J, Miller JM. Post-exposure treatment attenuates noise-induced hearing loss. Neuroscience. 2005; 134(2):633–42.
Article9. Henderson D, Bielefeld EC, Harris KC, Hu BH. The role of oxidative stress in noise-induced hearing loss. Ear Hear. 2006; Feb. 27(1):1–19.
Article10. Maeda Y, Fukushima K, Omichi R, Kariya S, Nishizaki K. Time courses of changes in phospho- and total- MAP kinases in the cochlea after intense noise exposure. PLoS One. 2013; 8(3):e58775.
Article11. Hodge DC, Price GR. Hearing damage risk criteria. In : Lipscomb DM, editor. Noise and audiology (perspectives in audiology series). Baltimore (ME): University Park Press;1978. p. 167–91.12. Clifford RE, Rogers RA. Impulse noise: theoretical solutions to the quandary of cochlear protection. Ann Otol Rhinol Laryngol. 2009; Jun. 118(6):417–27.
Article13. Starck J, Toppila E, Pyykko I. Impulse noise and risk criteria. Noise Health. 2003; Jul-Sep. 5(20):63–73.14. Akhmetzianov IM, Zinkin VI, Logatkin SM, Petreev IV, Kuznetsov SM, Dragan SP. Impulse noise at shooting from small arms and close combat weapon as the factor of military work. Voen Med Zh. 2012; Jun. 333(6):52–8.15. Rezaee M, Mojtahed M, Ghasemi M, Saedi B. Assessment of impulse noise level and acoustic trauma in military personnel. Trauma Mon. 2012; Jan. 16(4):182–7.
Article16. Lwow F, Jozkow P, Medras M. Occupational exposure to impulse noise associated with shooting. Int J Occup Saf Ergon. 2011; 17(1):69–77.
Article17. Forget P. Assessment of mean auditory hazard incurred by occupational exposure to impulse noise. Eur Ann Otorhinolaryngol Head Neck Dis. 2011; Jan. 128(1):14–7.
Article18. Sendowski I, Abaamrane L, Raffin F, Cros A, Clarencon D. Therapeutic efficacy of intra-cochlear administration of methylprednisolone after acoustic trauma caused by gunshot noise in guinea pigs. Hear Res. 2006; Nov. 221(1-2):119–27.
Article19. Flamme GA, Liebe K, Wong A. Estimates of the auditory risk from outdoor impulse noise. I: Firecrackers. Noise Health. 2009; Oct-Dec. 11(45):223–30.
Article20. Flamme GA, Wong A, Liebe K, Lynd J. Estimates of auditory risk from outdoor impulse noise. II: Civilian firearms. Noise Health. 2009; Oct-Dec. 11(45):231–42.
Article21. Wu CC, Young YH. Ten-year longitudinal study of the effect of impulse noise exposure from gunshot on inner ear function. Int J Audiol. 2009; Sep. 48(9):655–60.
Article22. Abaamrane L, Raffin F, Gal M, Avan P, Sendowski I. Long-term administration of magnesium after acoustic trauma caused by gunshot noise in guinea pigs. Hear Res. 2009; Jan. 247(2):137–45.
Article23. Levine S, Hofstetter P, Zheng XY, Henderson D. Duration and peak level as co-factors in hearing loss from exposure to impact noise. Scand Audiol Suppl. 1998; 48:27–36.24. Danielson R, Henderson D, Gratton MA, Bianchi L, Salvi R. The importance of “temporal pattern” in traumatic impulse noise exposures. J Acoust Soc Am. 1991; Jul. 90(1):209–18.
Article25. Pelausa EO, Abel SM, Simard J, Dempsey I. Prevention of noise-induced hearing loss in the Canadian military. J Otolaryngol. 1995; Oct. 24(5):271–80.26. Suvorov GA, Denisov EI, Antipin VG, Kharitonov VI, Starck Iu, Pyykko I, et al. Effects of peak levels and number of noise impulses on hearing among forge hammering workers. Med Tr Prom Ekol. 2002; (12):12–6.27. Neitzel R, Seixas N. The effectiveness of hearing protection among construction workers. J Occup Environ Hyg. 2005; Apr. 2(4):227–38.
Article28. Pourbakht A, Yamasoba T. Cochlear damage caused by continuous and intermittent noise exposure. Hear Res. 2003; Apr. 178(1-2):70–8.
Article29. Nilsson P, Rydmarker S, Grenner J. Impulse noise and continuous noise of equivalent frequency spectrum and total sound energy. Acta Otolaryngol Suppl. 1987; 441:45–58.30. Kil J, Pierce C, Tran H, Gu R, Lynch ED. Ebselen treatment reduces noise induced hearing loss via the mimicry and induction of glutathione peroxidase. Hear Res. 2007; Apr. 226(1-2):44–51.
Article31. Lynch ED, Gu R, Pierce C, Kil J. Ebselen-mediated protection from single and repeated noise exposure in rat. Laryngoscope. 2004; Feb. 114(2):333–7.
Article32. Fechter LD, Klis SF, Shirwany NA, Moore TG, Rao DB. Acrylonitrile produces transient cochlear function loss and potentiates permanent noise-induced hearing loss. Toxicol Sci. 2003; Sep. 75(1):117–23.
Article33. Rabinowitz PM, Pierce Wise J Sr, Hur Mobo B, Antonucci PG, Powell C, Slade M. Antioxidant status and hearing function in noise-exposed workers. Hear Res. 2002; Nov. 173(1-2):164–71.
Article34. Yamashita D, Jiang HY, Schacht J, Miller JM. Delayed production of free radicals following noise exposure. Brain Res. 2004; Sep. 1019(1-2):201–9.
Article35. Elsayed NM. Toxicology of blast overpressure. Toxicology. 1997; Jul. 121(1):1–15.
Article36. Huie RE, Padmaja S. The reaction of no with superoxide. Free Radic Res Commun. 1993; 18(4):195–9.
Article37. Linss V, Emmerich E, Richter F, Linss W. Is there a close relationship between changes in amplitudes of distortion product otoacoustic emissions and hair cell damage after exposure to realistic industrial noise in guinea pigs. Eur Arch Otorhinolaryngol. 2005; Jun. 262(6):488–95.
Article38. Roberto M, Hamernik RP, Turrentine GA. Damage of the auditory system associated with acute blast trauma. Ann Otol Rhinol Laryngol Suppl. 1989; May. 140:23–34.
Article39. Henderson D, McFadden SL, Liu CC, Hight N, Zheng XY. The role of antioxidants in protection from impulse noise. Ann N Y Acad Sci. 1999; Nov. 884(1):368–80.
Article40. Chi FL, Yang MQ, Zhou YD, Wang B. Therapeutic efficacy of topical application of dexamethasone to the round window niche after acoustic trauma caused by intensive impulse noise in guinea pigs. J Laryngol Otol. 2011; Jul. 125(7):673–85.
Article41. Chang MY, Rhee J, Kim SH, Kim YH. The protective effect of Egb 761 against 3-nitropropionic acid-induced hearing loss: the role of sirtuin 1. Clin Exp Otorhinolaryngol. 2018; Mar. 11(1):9–16.
Article42. Tian C, Kim YH, Kim YC, Park KT, Kim SW, Kim YJ, et al. Korean red ginseng ameliorates acute 3-nitropropionic acid-induced cochlear damage in mice. Neurotoxicology. 2013; Jan. 34:42–50.
Article43. Kim YH, Song JJ, Kim YC, Park KT, Lee JH, Choi JM, et al. Geranylgeranylacetone ameliorates acute cochlear damage caused by 3-nitropropionic acid. Neurotoxicology. 2010; Jun. 31(3):317–25.
Article44. Ohinata Y, Yamasoba T, Schacht J, Miller JM. Glutathione limits noiseinduced hearing loss. Hear Res. 2000; Aug. 146(1-2):28–34.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Bilateral Cochlear Implantation in Four Children
- Effect of Korean Red Ginseng Saponin on Cochlear Damage Induced by Noise Exposure
- Effect of Saponin, Allopurinol and Nimodipine on Noise Induced Treshold Changes of ABRs in Guinea Pigs
- Updates in Noise Induced Hearing Loss
- Morphological Change of Mouse Inner Ear Hair Cells after Noise Exposure