J Vet Sci.
2019 Jan;20(1):79-86. 10.4142/jvs.2019.20.1.79.
Iodixanol supplementation during sperm cryopreservation improves protamine level and reduces reactive oxygen species of canine sperm
- Affiliations
-
- 1Department of Theriogenology and Biotechnology, College of Veterinary Medicine, Seoul National University, Seoul 08826, Korea. bclee@snu.ac.kr
- KMID: 2434781
- DOI: http://doi.org/10.4142/jvs.2019.20.1.79
Abstract
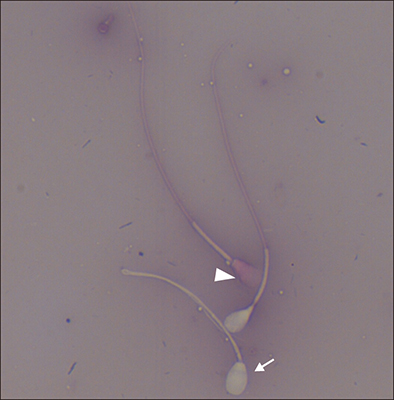
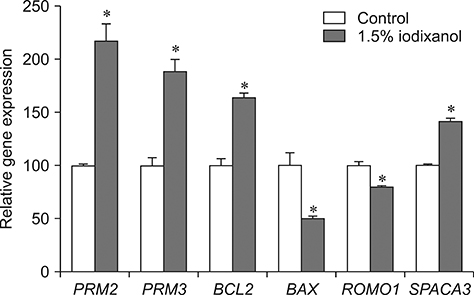
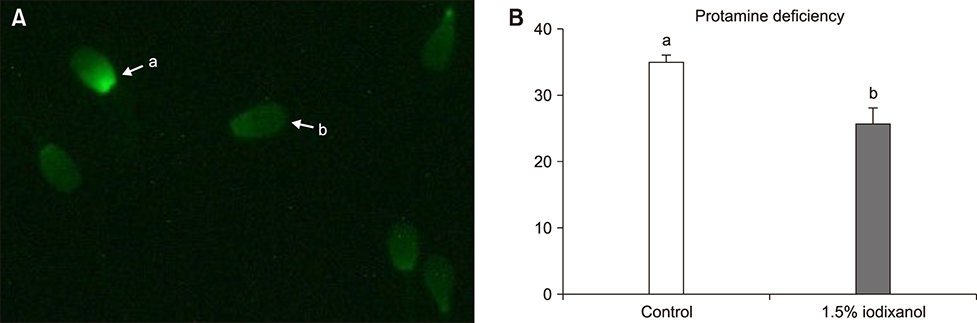
- The objective of this study was to analyze the protective effects of iodixanol on dog spermatozoa during cryopreservation. The optimal concentration of iodixanol, 1.5%, was determined using fresh spermatozoa and was applied in the following experiments. The 1.5% iodixanol group showed significantly increased sperm motility from that in the control (p < 0.05). Lower mitochondrial reactive oxygen species (ROS) modulator (ROMO1) and pro-apoptotic gene (BAX) expressions, together with higher expressions of protamine-2 (PRM2), protamine-3 (PRM3), anti-apoptotic B-cell lymphoma-2 (BCL2), and sperm acrosome associated-3 (SPACA3) genes were detected in the iodixanol-treated group. In addition, decreased protamine deficiency and cryocapacitation were observed in the treatment group. Our results show that supplementation with 1.5% iodixanol is ideal for reducing production of ROS and preventing detrimental effects during the canine sperm cryopreservation process, effects manifested as increased motility and reduced cryocapacitation in frozen-thawed spermatozoa.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Reactive Oxygen Species Modulator 1 (ROMO1), a New Potential Target for Cancer Diagnosis and Treatment
Mohammad Amin Amini, Seyed Saman Talebi, Jamshid Karimi
Chonnam Med J. 2019;55(3):136-143. doi: 10.4068/cmj.2019.55.3.136.
Reference
-
1. Agarwal A, Virk G, Ong C, du Plessis SS. Effect of oxidative stress on male reproduction. World J Mens Health. 2014; 32:1–17.
Article2. Aitken RJ. Sperm function tests and fertility. Int J Androl. 2006; 29:69–75.
Article3. Aitken RJ, Baker MA, Nixon B. Are sperm capacitation and apoptosis the opposite ends of a continuum driven by oxidative stress? Asian J Androl. 2015; 17:633–639.
Article4. Chenoweth P, Lorton S. Animal Andrology: Theories and Applications. Wallingford: Centre for Agriculture and Bioscience International (CABI);2014.5. Cirit Ü, Bağiş H, Demir K, Agca C, Pabuccuoğlu S, Varişli Ö, Clifford-Rathert C, Agca Y. Comparison of cryoprotective effects of iodixanol, trehalose and cysteamine on ram semen. Anim Reprod Sci. 2013; 139:38–44.
Article6. De Jonge CJ, Barratt CLR. The Sperm Cell: Production, Maturation, Fertilization, Regeneration. Cambridge: Cambridge University Press;2017.7. Ehmcke J, Schlatt S. Animal models for fertility preservation in the male. Reproduction. 2008; 136:717–723.
Article8. England GC, Millar KM. The ethics and role of AI with fresh and frozen semen in dogs. Reprod Domest Anim. 2008; 43:Suppl 2. 165–171.
Article9. Esterhuizen AD, Franken DR, Lourens JGH, Prinsloo E, van Rooyen LH. Sperm chromatin packaging as an indicator of in-vitro fertilization rates. Hum Reprod. 2000; 15:657–661.
Article10. Farstad W. Artificial insemination in dogs. In : England GCW, von Heimendahl A, editors. BSAVA Manual of Canine and Feline Reproduction and Neonatology. 2nd ed. Gloucester: British Small Animal Veterinary Association;2010. p. 80–88.11. Flinck A, Gottfridsson B. Experiences with iohexol and iodixanol during cardioangiography in an unselected patient population. Int J Cardiol. 2001; 80:143–151.
Article12. Flores E, Ramió-Lluch L, Bucci D, Fernández-Novell JM, Peña A, Rodríguez-Gil JE. Freezing-thawing induces alterations in histone H1-DNA binding and the breaking of protein-DNA disulfide bonds in boar sperm. Theriogenology. 2011; 76:1450–1464.
Article13. Giraud MN, Motta C, Boucher D, Grizard G. Membrane fluidity predicts the outcome of cryopreservation of human spermatozoa. Hum Reprod. 2000; 15:2160–2164.
Article14. Grzmil P, Boinska D, Kleene KC, Adham I, Schlüter G, Kämper M, Buyandelger B, Meinhardt A, Wolf S, Engel W. Prm3, the fourth gene in the mouse protamine gene cluster, encodes a conserved acidic protein that affects sperm motility. Biol Reprod. 2008; 78:958–967.
Article15. Guthrie HD, Welch GR. Effects of reactive oxygen species on sperm function. Theriogenology. 2012; 78:1700–1708.
Article16. Hammadeh ME, Dehn C, Hippach M, Zeginiadou T, Stieber M, Georg T, Rosenbaum P, Schmidt W. Comparison between computerized slow-stage and static liquid nitrogen vapour freezing methods with respect to the deleterious effect on chromatin and morphology of spermatozoa from fertile and subfertile men. Int J Androl. 2001; 24:66–72.
Article17. Jin JX, Lee S, Khoirinaya C, Oh A, Kim GA, Lee BC. Supplementation with spermine during in vitro maturation of porcine oocytes improves early embryonic development after parthenogenetic activation and somatic cell nuclear transfer. J Anim Sci. 2016; 94:963–970.
Article18. Kasimanickam VR, Kasimanickam RK, Rogers HA. Immunolocalization of retinoic acid receptor-alpha, -beta, and -gamma, in bovine and canine sperm. Theriogenology. 2013; 79:1010–1018.
Article19. Kim S, Hooper S, Agca C, Agca Y. Post-thaw ATP supplementation enhances cryoprotective effect of iodixanol in rat spermatozoa. Reprod Biol Endocrinol. 2016; 14:5.
Article20. Mandal A, Klotz KL, Shetty J, Jayes FL, Wolkowicz MJ, Bolling LC, Coonrod SA, Black MB, Diekman AB, Haystead TA, Flickinger CJ, Herr JC. SLLP1, a unique, intra-acrosomal, non-bacteriolytic, c lysozyme-like protein of human spermatozoa. Biol Reprod. 2003; 68:1525–1537.
Article21. Matás C, Decuadro G, Martínez-Miró S, Gadea J. Evaluation of a cushioned method for centrifugation and processing for freezing boar semen. Theriogenology. 2007; 67:1087–1091.
Article22. McLaughlin EA, Ford WCL, Hull MGR. The contribution of the toxicity of a glycerol-egg yolk-citrate cryopreservative to the decline in human sperm motility during cryopreservation. J Reprod Fertil. 1992; 95:749–754.
Article23. Miguel-Jiménez S, Mogas T, Peña AI, Tamargo C, Hidalgo CO, Muiño R, Rodríguez-Gil JE, Morató R. Post-thaw changes in sperm membrane and ROS following cryopreservation of dairy bull semen using four different commercial extenders. In : Physiology of Reproduction in Male and Semen Technology (Abstracts A191E to A205E): 32nd Meeting of the European Embryo Transfer Association (AETE); 9–10 August 2016; Barcelona, Spain.24. Nagashima JB, Sylvester SR, Nelson JL, Cheong SH, Mukai C, Lambo C, Flanders JA, Meyers-Wallen VN, Songsasen N, Travis AJ. Live births from domestic dog (Canis familiaris) embryos produced by in vitro fertilization. PLoS One. 2015; 10:e0143930.25. Peña AI, Barrio M, Becerra JJ, Quintela LA, Herradón PG. Motile sperm subpopulations in frozen-thawed dog semen: changes after incubation in capacitating conditions and relationship with sperm survival after osmotic stress. Anim Reprod Sci. 2012; 133:214–223.
Article26. Saragusty J, Gacitua H, Rozenboim I, Arav A. Protective effects of iodixanol during bovine sperm cryopreservation. Theriogenology. 2009; 71:1425–1432.
Article27. Schneider S, Balbach M, Jikeli JF, Fietz D, Nettersheim D, Jostes S, Schmidt R, Kressin M, Bergmann M, Wachten D, Steger K, Schorle H. Re-visiting the Protamine-2 locus: deletion, but not haploinsufficiency, renders male mice infertile. Sci Rep. 2016; 6:36764.
Article28. Schulte RT, Ohl DA, Sigman M, Smith GD. Sperm DNA damage in male infertility: etiologies, assays, and outcomes. J Assist Reprod Genet. 2010; 27:3–12.
Article29. Setyawan EMN, Kim MJ, Oh HJ, Kim GA, Jo YK, Lee SH, Choi YB, Lee BC. Maintaining canine sperm function and osmolyte content with multistep freezing protocol and different cryoprotective agents. Cryobiology. 2015; 71:344–349.
Article30. Setyawan EMN, Kim MJ, Oh HJ, Kim GA, Jo YK, Lee SH, Choi YB, Lee BC. Spermine reduces reactive oxygen species levels and decreases cryocapacitation in canine sperm cryopreservation. Biochem Biophys Res Commun. 2016; 479:927–932.
Article31. Shin JA, Chung JS, Cho SH, Kim HJ, Yoo YD. Romo1 expression contributes to oxidative stress-induced death of lung epithelial cells. Biochem Biophys Res Commun. 2013; 439:315–320.
Article32. Simon HU, Haj-Yehia A, Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. 2000; 5:415–418.33. Simon L, Murphy K, Shamsi MB, Liu L, Emery B, Aston KI, Hotaling J, Carrell DT. Paternal influence of sperm DNA integrity on early embryonic development. Hum Reprod. 2014; 29:2402–2412.
Article34. Swami DS, Kumar P, Malik RK, Saini M, Kumar D, Jan MH. The cryoprotective effect of iodixanol in buffalo semen cryopreservation. Anim Reprod Sci. 2017; 179:20–26.
Article35. Tremellen K. Oxidative stress and male infertility–a clinical perspective. Hum Reprod Update. 2008; 14:243–258.
Article36. Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003; 552:335–344.
Article37. Van den Berghe F, Paris MCJ, Briggs MB, Farstad WK, Paris DBBP. A two-step dilution tris-egg yolk extender containing Equex STM significantly improves sperm cryopreservation in the African wild dog (Lycaon pictus). Cryobiology. 2018; 80:18–25.
Article38. Zalata AA, Mokhtar N, Atwa A, Khaled M, Shaker OG. The role of protamine 2 gene expression and caspase 9 activity in male infertility. J Urol. 2016; 195:796–800.
Article39. Zini A, Boman JM, Belzile E, Ciampi A. Sperm DNA damage is associated with an increased risk of pregnancy loss after IVF and ICSI: systematic review and metaanalysis. Hum Reprod. 2008; 23:2663–2668.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of L-carnitine and Acetylcarnitine on Sperm Parameters in vitro
- Effects of Anti-oxidants in Freezing and Thawing of Sperm
- Antioxidative Effect of Rebamipide in Cryopreservation and Thawing of Sperm
- Supplementation of cryoprotective extender with resveratrol decreases apoptosis index and reactive oxygen species levels in post-thaw dog sperm
- The relationship between reactive oxygen species, DNA fragmentation, and sperm parameters in human sperm using simplified sucrose vitrification with or without triple antioxidant supplementation