Korean J Ophthalmol.
2019 Feb;33(1):16-25. 10.3341/kjo.2018.0111.
Ocular Surface Reconstruction Using Circumferentially-trephined Autologous Oral Mucosal Graft Transplantation in Limbal Stem Cell Deficiency
- Affiliations
-
- 1Department of Ophthalmology, Seoul National University College of Medicine, Seoul, Korea. kmk9@snu.ac.kr
- 2Laboratory of Ocular Regenerative Medicine and Immunology, Seoul Artificial Eye Center, Seoul National University Hospital Biomedical Research Institute, Seoul, Korea.
- KMID: 2434301
- DOI: http://doi.org/10.3341/kjo.2018.0111
Abstract
- PURPOSE
To investigate the effects of transplantation of a circumferentially-trephined autologous oral mucosal graft using a vacuum trephine on ocular surface reconstruction in patients with limbal stem cell deficiency.
METHODS
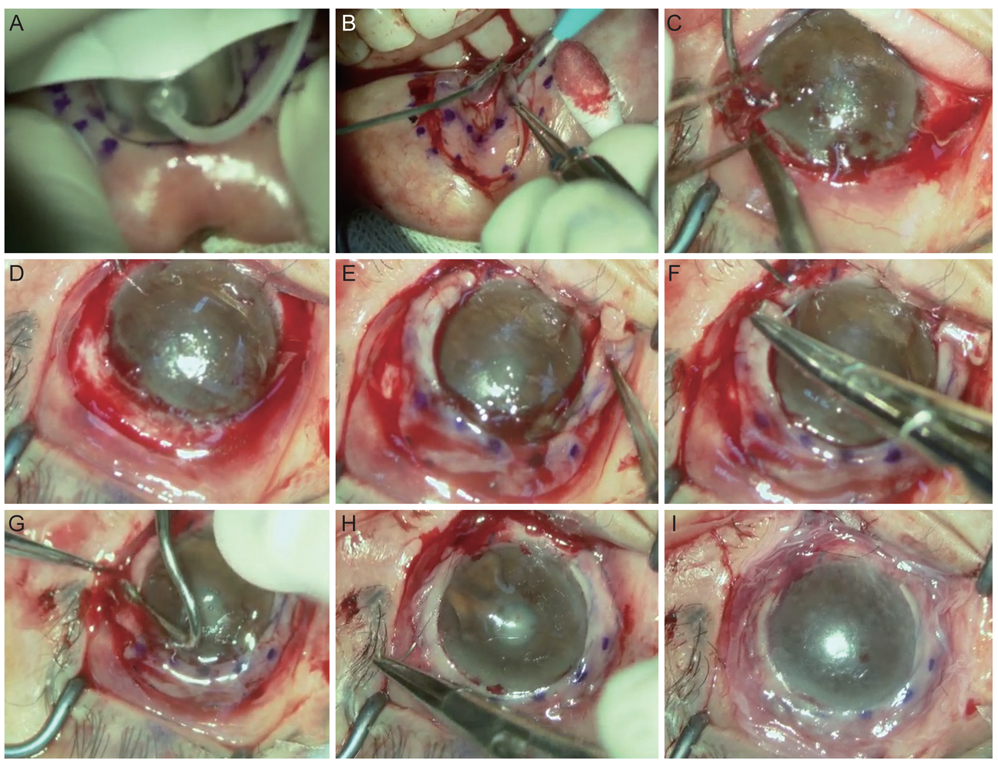
Patients with a limbal stem cell deficiency who underwent transplantation of autologous oral mucosal graft performed by a particular surgeon in Seoul National University Hospital were included. The medical records of these five patients were retrospectively reviewed. The lower labial mucosal graft inside the inferior lip was trephined to a depth of 250 µm using a donor vacuum trephine with a 9-mm diameter. Outside markings were made using a 14-mm intraoperative keratometer. The oral mucosal graft was dissected under a microscope using a Beaver mini-blade as either a ring or a crescent-shaped strip with a 5-mm width. The mucosal graft was transplanted onto the limbus in the limbal-deficient eye. Best-corrected visual acuity and corneal status were measured during the follow-up period.
RESULTS
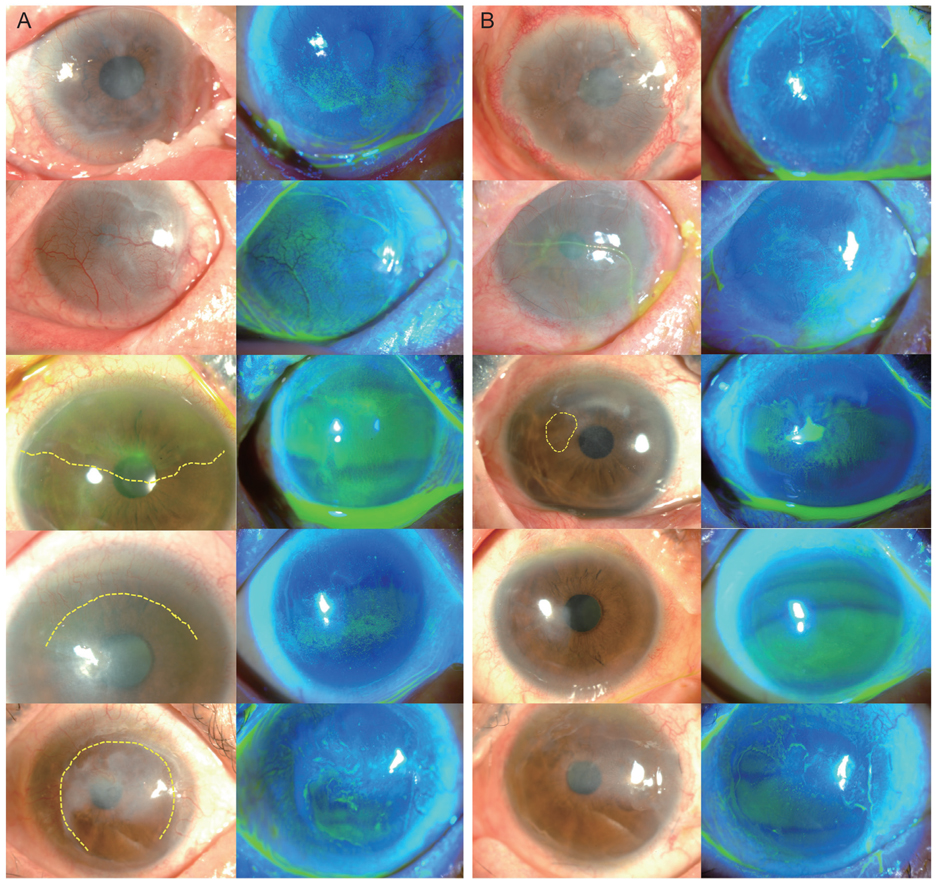
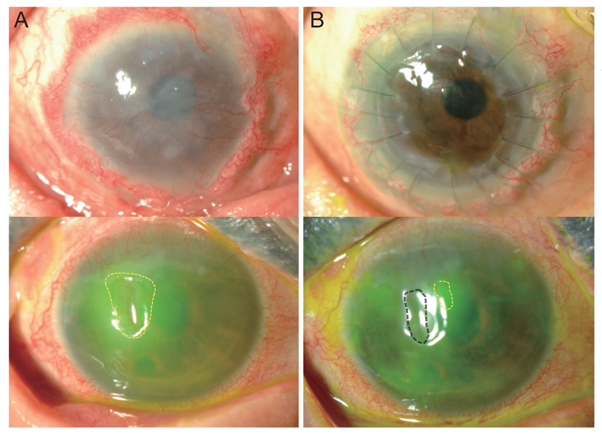
Four patients were diagnosed with Stevens-Johnson syndrome and one was diagnosed with atopy-associated immune keratitis. The mean follow-up period was 10.4 ± 2.9 months. After 4 months, visual acuity improved in all patients, and the mean improvement in logarithm of the minimum angle of resolution visual acuity was 0.526 ± 0.470 (range, 0.15 to 1.10). Corneal surface erosion and neovascularization decreased in four patients, and stromal opacity decreased in two patients. The engraftments maintained ocular surface stabilization in four of the five patients at the last follow-up.
CONCLUSIONS
Transplantation of circumferential autologous oral mucosal grafts may be effective for the treatment of limbal stem cell deficiency.
MeSH Terms
Figure
Reference
-
1. Sun TT, Tseng SC, Lavker RM. Location of corneal epithelial stem cells. Nature. 2010; 463:E10–E11.
Article2. Puangsricharern V, Tseng SC. Cytologic evidence of corneal diseases with limbal stem cell deficiency. Ophthalmology. 1995; 102:1476–1485.3. Le Q, Xu J, Deng SX. The diagnosis of limbal stem cell deficiency. Ocul Surf. 2018; 16:58–69.
Article4. Kenyon KR. Limbal autograft transplantation for chemical and thermal burns. Dev Ophthalmol. 1989; 18:53–58.
Article5. Tsai RJ, Tseng SC. Human allograft limbal transplantation for corneal surface reconstruction. Cornea. 1994; 13:389–400.
Article6. Tsubota K, Toda I, Saito H, et al. Reconstruction of the corneal epithelium by limbal allograft transplantation for severe ocular surface disorders. Ophthalmology. 1995; 102:1486–1496.
Article7. Holland EJ. Epithelial transplantation for the management of severe ocular surface disease. Trans Am Ophthalmol Soc. 1996; 94:677–743.
Article8. Solomon A, Ellies P, Anderson DF, et al. Long-term outcome of keratolimbal allograft with or without penetrating keratoplasty for total limbal stem cell deficiency. Ophthalmology. 2002; 109:1159–1166.
Article9. Pellegrini G, Traverso CE, Franzi AT, et al. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 1997; 349:990–993.
Article10. Zhao Y, Ma L. Systematic review and meta-analysis on transplantation of ex vivo cultivated limbal epithelial stem cell on amniotic membrane in limbal stem cell deficiency. Cornea. 2015; 34:592–600.
Article11. Koizumi N, Inatomi T, Suzuki T, et al. Cultivated corneal epithelial stem cell transplantation in ocular surface disorders. Ophthalmology. 2001; 108:1569–1574.12. Shimazaki J, Aiba M, Goto E, et al. Transplantation of human limbal epithelium cultivated on amniotic membrane for the treatment of severe ocular surface disorders. Ophthalmology. 2002; 109:1285–1290.13. Utheim TP. Concise review: transplantation of cultured oral mucosal epithelial cells for treating limbal stem cell deficiency-current status and future perspectives. Stem Cells. 2015; 33:1685–1695.
Article14. Nishida K, Yamato M, Hayashida Y, et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med. 2004; 351:1187–1196.
Article15. Takeda K, Nakamura T, Inatomi T, et al. Ocular surface reconstruction using the combination of autologous cultivated oral mucosal epithelial transplantation and eyelid surgery for severe ocular surface disease. Am J Ophthalmol. 2011; 152:195–201.
Article16. Nakamura T, Takeda K, Inatomi T, et al. Long-term results of autologous cultivated oral mucosal epithelial transplantation in the scar phase of severe ocular surface disorders. Br J Ophthalmol. 2011; 95:942–946.
Article17. Kim YJ, Lee HJ, Ryu JS, et al. Prospective clinical trial of corneal reconstruction with biomaterial-free cultured oral mucosal epithelial cell sheets. Cornea. 2018; 37:76–83.
Article18. Sangwan VS, Basu S, MacNeil S, Balasubramanian D. Simple limbal epithelial transplantation (SLET): a novel surgical technique for the treatment of unilateral limbal stem cell deficiency. Br J Ophthalmol. 2012; 96:931–934.
Article19. Li J, O'Reilly N, Sheha H, et al. Correlation between ocular Demodex infestation and serum immunoreactivity to Bacillus proteins in patients with Facial rosacea. Ophthalmology. 2010; 117:870–877.
Article20. Tsubota K, Goto E, Fujita H, et al. Treatment of dry eye by autologous serum application in Sjögren's syndrome. Br J Ophthalmol. 1999; 83:390–395.
Article21. Lemp MA. Report of the National Eye Institute/Industry workshop on Clinical Trials in Dry Eyes. CLAO J. 1995; 21:221–232.22. Inatomi T, Nakamura T, Koizumi N, et al. Midterm results on ocular surface reconstruction using cultivated autologous oral mucosal epithelial transplantation. Am J Ophthalmol. 2006; 141:267–275.
Article23. Shimazaki J, Shimmura S, Fujishima H, Tsubota K. Association of preoperative tear function with surgical outcome in severe Stevens-Johnson syndrome. Ophthalmology. 2000; 107:1518–1523.
Article24. Fu Y, Liu J, Tseng SC. Oral mucosal graft to correct lid margin pathologic features in cicatricial ocular surface diseases. Am J Ophthalmol. 2011; 152:600–608.
Article25. Iyer G, Pillai VS, Srinivasan B, et al. Mucous membrane grafting for lid margin keratinization in Stevens-Johnson syndrome: results. Cornea. 2010; 29:146–151.
Article26. Shore JW, Foster CS, Westfall CT, Rubin PA. Results of buccal mucosal grafting for patients with medically controlled ocular cicatricial pemphigoid. Ophthalmology. 1992; 99:383–395.
Article27. Nakamura T, Endo K, Cooper LJ, et al. The successful culture and autologous transplantation of rabbit oral mucosal epithelial cells on amniotic membrane. Invest Ophthalmol Vis Sci. 2003; 44:106–116.
Article28. Nakamura T, Kinoshita S. Ocular surface reconstruction using cultivated mucosal epithelial stem cells. Cornea. 2003; 22:S75–S80.
Article29. Madhira SL, Vemuganti G, Bhaduri A, et al. Culture and characterization of oral mucosal epithelial cells on human amniotic membrane for ocular surface reconstruction. Mol Vis. 2008; 14:189–196.30. Chen HC, Chen HL, Lai JY, et al. Persistence of transplanted oral mucosal epithelial cells in human cornea. Invest Ophthalmol Vis Sci. 2009; 50:4660–4668.
Article31. Sotozono C, Inatomi T, Nakamura T, et al. Visual improvement after cultivated oral mucosal epithelial transplantation. Ophthalmology. 2013; 120:193–200.
Article32. Liu J, Sheha H, Fu Y, et al. Oral mucosal graft with amniotic membrane transplantation for total limbal stem cell deficiency. Am J Ophthalmol. 2011; 152:739–747.
Article33. Han ES, Wee WR, Lee JH, Kim MK. Long-term outcome and prognostic factor analysis for keratolimbal allografts. Graefes Arch Clin Exp Ophthalmol. 2011; 249:1697–1704.
Article34. Ilari L, Daya SM. Long-term outcomes of keratolimbal allograft for the treatment of severe ocular surface disorders. Ophthalmology. 2002; 109:1278–1284.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- New Strategy of Ocular Surface Disease: Ocular Surface Reconstruction Using Amniotic Membrane and Limbal Stem Cell Transplantation
- Living Related Conjunctival Limbal Allograft
- Transplantation of in vivo Cultivated Limbal Corneal Epithelial Cells with Total Limbal Stem Cell Deficiency
- A Case of Ocular Surface Reconstruction Using Nasal and Oral Mucosa Autograft
- Amniotic Membrane Transplanted in Conjunctiva as a Mesenchymal Stem Cells Carrier for Limbal Stem Cell Deficiency