Yeungnam Univ J Med.
2018 Dec;35(2):192-198. 10.12701/yujm.2018.35.2.192.
Antiepileptic and anti-neuroinflammatory effects of red ginseng in an intrahippocampal kainic acid model of temporal lobe epilepsy demonstrated by electroencephalography
- Affiliations
-
- 1Department of Pediatrics, Yeungnam University College of Medicine, Daegu, Korea. sysnow88@hanmail.net
- 2Department of Neurosurgery, Yeungnam University College of Medicine, Daegu, Korea.
- 3Laboratory Animal Center, Daegu-Gyeongbuk Medical Innovation Foundation, Daegu, Korea.
- KMID: 2433738
- DOI: http://doi.org/10.12701/yujm.2018.35.2.192
Abstract
- BACKGROUND
Chronic inflammation can lower the seizure threshold and have influence on epileptogenesis. The components of red ginseng (RG) have anti-inflammatory effects. The abundance of peripherally derived immune cells in resected epileptic tissue suggests that the immune system is a potential target for anti-epileptogenic therapies. The present study used continuous electroencephalography (EEG) to evaluate the therapeutic efficacy of RG in intrahippocampal kainic acid (IHKA) animal model of temporal lobe epilepsy.
METHODS
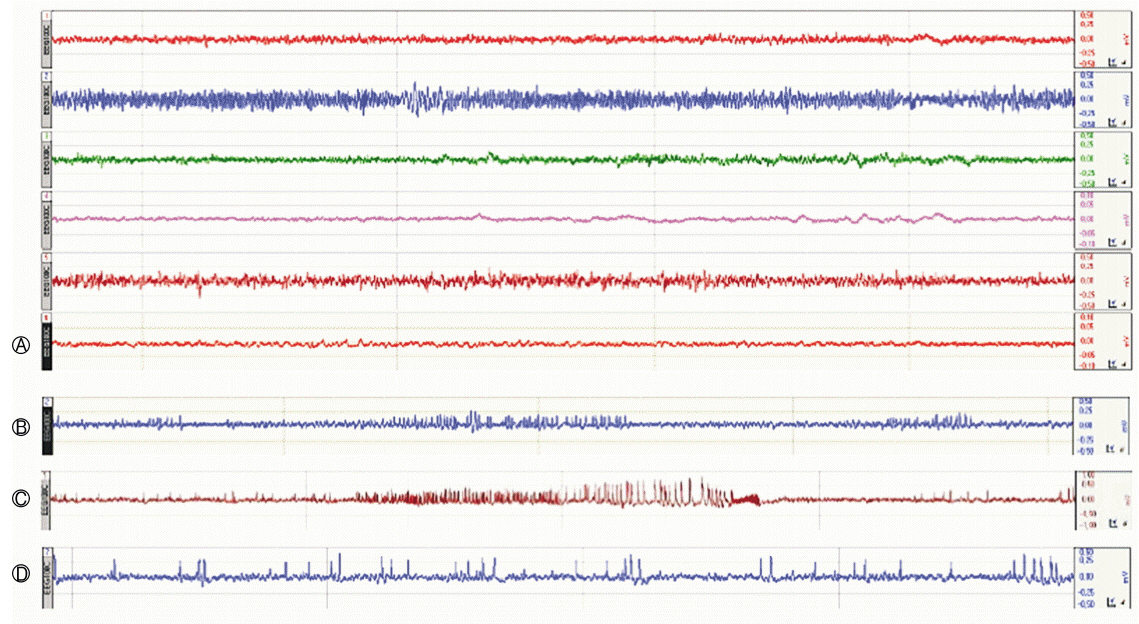
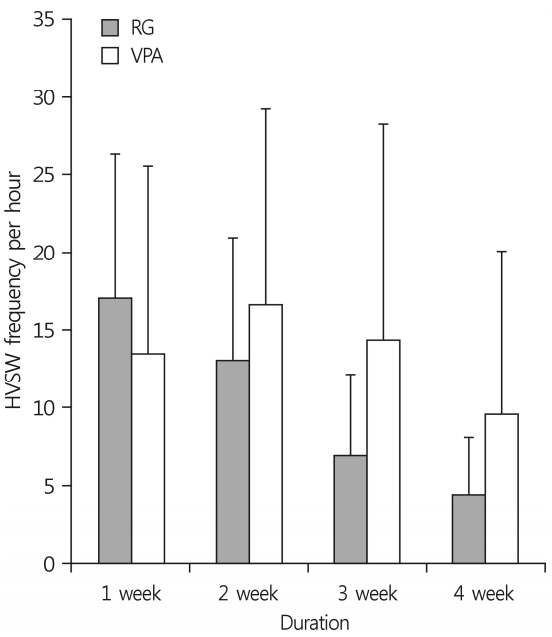
Prolonged status epilepticus (SE) was induced in 7-week-old C57BL/6J mice via stereotaxic injection of kainic acid (KA, 150 nL; 1 mg/mL) into the right CA3/dorsal hippocampus. The animals were implanted electrodes and monitored for spontaneous seizures. Following the IHKA injections, one group received treatments of RG (250 mg/kg/day) for 4 weeks (RG group, n=7) while another group received valproic acid (VPA, 30 mg/kg/day) (VPA group, n=7). Laboratory findings and pathological results were assessed at D29 and continuous (24 h/week) EEG monitoring was used to evaluate high-voltage sharp waves on D7, D14, D21, and D28.
RESULTS
At D29, there were no differences between the groups in liver function test but RG group had higher blood urea nitrogen levels. Immunohistochemistry analyses revealed that RG reduced the infiltration of immune cells into the brain and EEG analyses showed that it had anticonvulsant effects.
CONCLUSION
Repeated treatments with RG after IHKA-induced SE decreased immune cell infiltration into the brain and resulted in a marked decrease in electrographic seizures. RG had anticonvulsant effects that were similar to those of VPA without serious side effects.
Keyword
MeSH Terms
Figure
Reference
-
1. Kienzler-Norwood F, Costard L, Sadangi C, Müller P, Neubert V, Bauer S, et al. A novel animal model of acquired human te mporal lobe epilepsy based on the simultaneous administration of kainic acid and lorazepam. Epilepsia. 2017; 58:222–30.
Article2. Lévesque M, Avoli M. The kainic acid model of temporal lobe epilepsy. Neurosci Biobehav Rev. 2013; 37:2887–99.
Article3. Engel J Jr, McDermott MP, Wiebe S, Langfitt JT, Stern JM, Dewar S, et al. Early surgical therapy for drug-resistant temporal lobe epilepsy: a randomized trial. JAMA. 2012; 307:922–30.
Article4. Babiker LB, Gadkariem EA, Alashban RM, ALjohar HI. Investigation of stability of Korean ginseng in herbal drug product. Am J Appl Sci. 2014; 11:160–70.
Article5. Kim SJ, Choi S, Kim M, Park C, Kim GL, Lee SO, et al. Effect of Korean Red Ginseng extracts on drug-drug interactions. J Ginseng Res. 2018; 42:370–8.
Article6. Kim KH, Lee D, Lee HL, Kim CE, Jung K, Kang KS. Beneficial effects of Panax ginseng for the treatment and prevention of neurodegenerative diseases: past findings and future directions. J Ginseng Res. 2018; 42:239–47.
Article7. Choi JH, Jang M, Nah SY, Oh S, Cho IH. Multitarget effects of Korean Red Ginseng in animal model of Parkinson’s disease: antiapoptosis, antioxidant, antiinflammation, and maintenance of blood-brain barrier integrity. J Ginseng Res. 2018; 42:379–88.
Article8. Twele F, Töllner K, Brandt C, Löscher W. Significant effects of sex, strain, and anesthesia in the intrahippocampal kainate mouse model of mesial temporal lobe epilepsy. Epilepsy Behav. 2016; 55:47–56.
Article9. Conte F, Legros B, Van Paesschen W, Avbersek A, Muglia P, Depondt C. Long-term seizure outcomes in patients with drug resistant epilepsy. Seizure. 2018; 62:74–8.
Article10. Andres-Mach M, Zagaja M, Haratym-Maj A, Rola R, Maj M, Haratym J, et al. A long-term treatment with arachidonyl-2'-chloroethylamide combined with valproate increases neurogenesis in a mouse pilocarpine model of epilepsy. Int J Mol Sci. 2017; 18:pii: E900.
Article11. Thom M. Hippocampal sclerosis in epilepsy: a neuropathology review. Neuropathol Appl Neurobiol. 2014; 40:520–43.
Article12. Misra UK, Dubey D, Kalita J. Comparison of lacosamide versus sodium valproate in status epilepticus: a pilot study. Epilepsy Behav. 2017; 76:110–3.
Article13. Curia G, Lucchi C, Vinet J, Gualtieri F, Marinelli C, Torsello A, et al. Pathophysiogenesis of mesial temporal lobe epilepsy: is prevention of damage antiepileptogenic? Curr Med Chem. 2014; 21:663–88.
Article14. Bielefeld P, Sierra A, Encinas JM, Maletic-Savatic M, Anderson A, Fitzsimons CP. A standardized protocol for stereotaxic intrahippocampal administration of kainic acid combined with electroencephalographic seizure monitoring in mice. Front Neurosci. 2017; 11:160.
Article15. Bouilleret V, Ridoux V, Depaulis A, Marescaux C, Nehlig A, Le Gal La Salle G. Recurrent seizures and hippocampal sclerosis following intrahippocampal kainate injection in adult mice: electroencephalography, histopathology and synaptic reorganization similar to mesial temporal lobe epilepsy. Neuroscience. 1999; 89:717–29.
Article16. Kim MK, Kang H, Baek CW, Jung YH, Woo YC, Choi GJ, et al. Antinociceptive and anti-inflammatory effects of ginsenoside Rf in a rat model of incisional pain. J Ginseng Res. 2018; 42:183–91.
Article17. Twele F, Töllner K, Brandt C, Löscher W. Significant effects of sex, strain, and anesthesia in the intrahippocampal kainate mouse model of mesial temporal lobe epilepsy. Epilepsy Behav. 2016; 55:47–56.
Article18. Lawthom C. Valproate and epilepsy: for women as well as men. Pract Neurol. 2018; 18:222–3.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Temporal lobe epilepsy caused by intrahippocampal calcified cysticercus: a case report
- Evaluation of Memory Impairment in Patients with Temporal Lobe Epilepsy Using the Wechsler Memory Scale
- Electroencephalographic, Behavioral and Pathologic Characteristics in Experimental Complex Partial Seizure Induced by Microinjection of Kainic Acid into the Unilateral Amygdala in the Rat
- Clinical Correlations between Duration of Epilepsy and Anticonvulsant Treatment Response in Temporal Lobe Epilepsy
- Beneficial Effects of Silibinin Against Kainic Acid-induced Neurotoxicity in the Hippocampus in vivo