Nat Prod Sci.
2018 Dec;24(4):272-283. 10.20307/nps.2018.24.4.272.
Nematicidal Compounds from the Leaves of Schinus terebinthifolius Against Root-knot Nematode, Meloidogyne incognita Infecting Tomato
- Affiliations
-
- 1Department of Pharmacognosy, Faculty of Pharmacy, Prince Sattam Bin Abdulaziz University, Al-Kharj, 11942, Saudi Arabia. fatma_maar@yahoo.com
- 2Department of Pharmacognosy, Faculty of Pharmacy, Mansoura University, Mansoura, 35516, Egypt.
- 3Department of Nematology, Plant Pathology Res. Inst., Agric. Res. Center, Giza, Egypt.
- KMID: 2432423
- DOI: http://doi.org/10.20307/nps.2018.24.4.272
Abstract
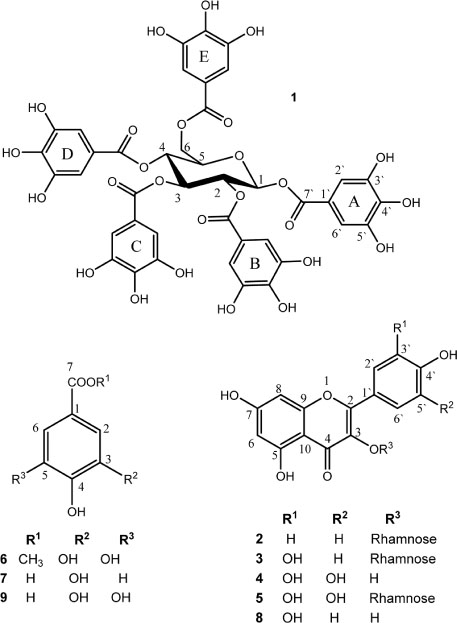
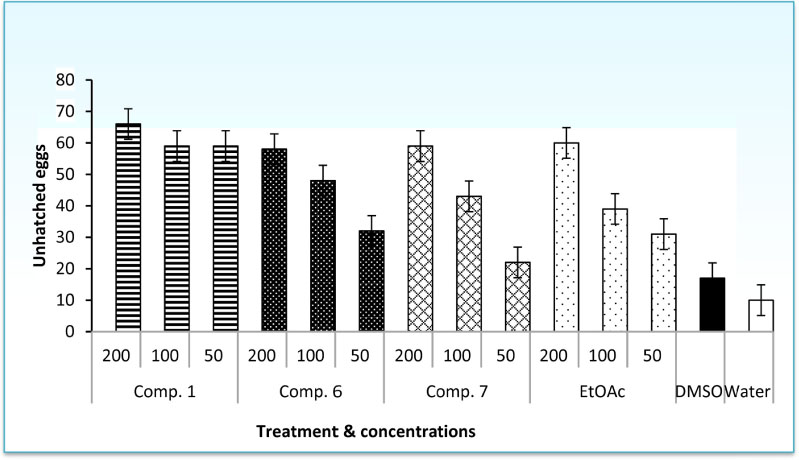
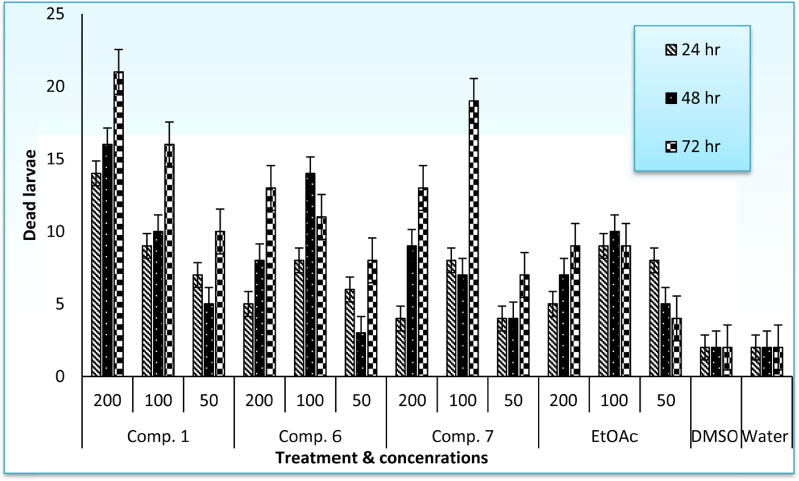
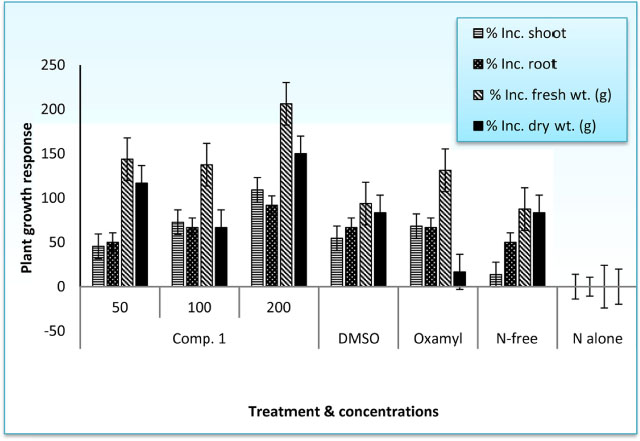
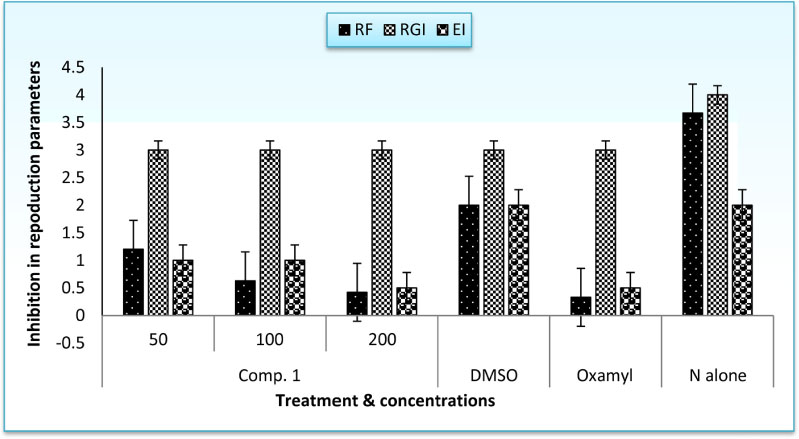
- The root-knot nematode, Meloidogyne incognita caused a serious damage to many plants. The phenolic components of the leaves of Schinus terebinthifolius were investigated as potential nematicidal agents for M. incognita. Nine compounds were isolated and characterized as viz., 1,2,3,4,6-pentagalloyl glucose (1), kaempferol-3-O-α-L-rhamnoside (Afzelin) (2), quercetin-3-O-α-L-rhamnoside (Quercetrin) (3), myricetin (4), myricetin-3-O-α-L-rhamnoside (Myricetrin) (5), methylgallate (6), protocatechuic acid (7), quercetin (8), and gallic acid (9) using nuclear magnetic resonance (NMR) spectroscopy. Compound 1 showed pronounced nematicidal activity compared to Oxamyl as a positive control. It showed the lowest eggs-hatchability (34%) and the highest mortality in nematode population (21% after 72 hours of treatment) at a concentration of 200 µg/mL. It exhibited the best suppressed total nematode population, root galling and number of eggmasses in infected tomato plants. The total carbohydrates and proteins were also significantly induced by 1 with reduction in total phenolics and increase in defense-related proteins. Thus, compound 1 could be a promising, more safe and effective natural nematicidal agent for the control of root-knot nematodes.
Keyword
MeSH Terms
Figure
Reference
-
1. Amorim MMR, Santos LC. Rev Bras Gincecol Obstet. 2003; 25:95–102.2. de Lima MR, Luna JD-S, dos Santos AF, de Andrade MCC, et al. J Ethnopharmacol. 2006; 105:137–147.3. Johann S, Pizzolatti M, Donnici CL, de Resende MA. Braz J Microbiol. 2007; 38:632–637.4. Bernardes NR, Heggdorne-Araújo M, Borges IFJC, Almeida FM, Amaral EP, Lasunskaia EB, Muzitano MF, Oliveira DB. Rev Bras Farmacogn. 2014; 24:644–650.5. Johann S, Silva DL, Martins CVB, Zani CL, Pizzolatti MG, Resende MA. World J Microbiol Biotechnol. 2008; 24:2459–2464.6. Johann S, Sá NP, Lima LARS, Cisalpino PS, Cota BB, Alves TMA, Siqueira EP, Zani CL. Ann Clin Microbiol Antimicrob. 2010; 9:30–36.7. Carvalho MG, Melo AGN, Aragao CFS, Raffin FN, Moura TFAL. Rev Bras Pl Med. 2013; 15:158–169.8. Fedel-Miyasato LES, Kassuya CAL, Auharek SA, Formagio ASN, Cardoso CAL, Mauro MO, Cunha-Laura AL, Monreal ACD, Vieira MC, Oliveira RJ. Rev Bras Farmacogn. 2014; 24:565–575.9. Quave CL, Plano LRW, Bennett BC. Planta Med. 2008; 74:43.10. Queires LC, Fauvel-Lafètve F, Terry S, De la Taille A, Kouyoumdjian JC, Chopin DK, Vacherot F, Rodrigues LE, Crépin M. Anticancer Res. 2006; 26:379–387.11. Cavalher-Machado SC, Rosas EC, Brito FA, Heringe AP, de Oliveira RR, Ka-Plan MA, Figueiredo MR, Henriques M. Int Immunopharmacol. 2008; 8:1552–1560.12. Santana JS, Lago PSJHG, Matsuo AL. Quim Nova. 2012; 35:2245–2248.13. Carvalho MG, Freire FD, Raffin FN, Aragão CFS, Moura TFAL. Chromatographia. 2009; 69:249–253.14. Farag SF. Bull Pharm Sci. 2008; 31:319–329.15. Heringer AP, Oliveira RR, Figueiredo MR, Kaplan MA. 30a reunião anual da sociedade brasileira de química. 2007.16. Hayashi T, Nagayama K, Arisawa M, Shimizu M, Suzuki S, Yoshizaki M, Morita N, Ferro E, Basualdo I, Berganza LH. J Nat Prod. 1989; 52:210–211.17. Morais TR, da Costa-Silva TA, Tempone AG, Borborema SET, Scotti MT, de Sousa RMF, Araujo ACC, de Oliveira A, de Morais SAL, Sartorelli P, Lago JHG. Molecules. 2014; 19:5761–5776.18. Campello JP, Marsaioli AJ. Phytochemistry. 1975; 14:2300–2302.19. Lloyd HA, Jaouni TM, Evans SL, Morton JF. Phytochemistry. 1977; 16:1301–1302.20. Sasser JN, Freckman DW. Veech JA, Dickson DW, editors. World perspective on nematology: The role of the society, in Vistas on Nematology. Hyattsville, Maryland: A Commemoration of the 25th Anniversary of the Society of Nematologists, Society of Nematologists, Inc.;1987. p. 7–14.21. Hussey RS, Barker KR. Plant Dis Reptr. 1973; 57:1925–1928.22. Goodey JB. Laboratory methods for work with plant and soil nematodes. London: Tech. Bull., 2, Min. Agric. Fish Ed;1957. p. 47.23. Taylor AL, Sasser JN. Biology, identification and control of root-knot Nematodes (Meloidogyne spp.). Raleigh. N.C.: Coop. Pub. Dept. Pl. pathol. North Carolina State Univ. and U.S. Agency Int. Dev.;1978. p. 111.24. Patel DJ, Patel HV, Patel SK, Patel BA. Indian J Pl Protect. 1993; 21:242–244.25. Maxwell DP, Bateman DF. Phytopathology. 1967; 57:132–136.
Article26. Bradford MM. Anal Biochem. 1976; 72:248–254.27. Thomas W, Dutcher RA. J Am Chem Soc. 1924; 46:1662–1669.28. Kaur C, Kapoor HC. Int J Food Sci Technol. 2002; 37:153–161.29. Amako A, Ghen GX, Asala K. Plant Cell Physiol. 1994; 53:497–507.30. Coseteng MY, Lee CY. J Food Sci. 1987; 52:985–989.31. Gomez KA, Gomez AA. Statistical Procedures for Agriculture Research. 2nd Ed. New York: John Wiley & Sons. Inc;1984. p. 101.32. Duncan DB. Biometrics. 1955; 11:1–42.33. Dos Santos RT, Hiramoto LL, Lago JHG, Sartorelli P, Tempone AG, Pinto EG, Lorenzi H. Quim Nova. 2012; 35:2229–2232.34. Da Silva MM, Iriguchi EKK, Kassuya CAL, Vieira MD-C, Foglio MA, de Carvalho JE, Ruiz ALTG, Souza KD-P, Formagio ASN. Rev Bras Farmacogn. 2017; 27:445–452.35. Ceruks M, Romoff P, Favero AO, Lago JHG. Quim Nova. 2007; 30:597–599.36. Agrawal PK. Studies in Organic Chemistry Carbon-13 NMR of Flavonoids. Amsterdam: Elsevier;1989. p. 334. p. 336. p. 338.37. Zhang Z, Liao L, Moore J, Wu T, Wan Z. Food Chem. 2009; 113:160–165.38. Gautam SK, Poddar AN. Int J Curr Microbiol App Sci. 2014; 3:470–478.39. El-beltagi HS, Farahat AA, Alsayed AA, Mahfoud NA. Not Bot Horti Agrobo. 2012; 40:132–142.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Use of Dactylaria brochopaga, a Predacious Fungus, for Managing Root-Knot Disease of Wheat (Triticum aestivum) Caused by Meloidogyne graminicola
- Draft Genome Sequence of Xylaria grammica EL000614, a Strain Producing Grammicin, a Potent Nematicidal Compound
- Draft Genome Sequence of Xylaria grammica EL000614, a Strain Producing Grammicin, a Potent Nematicidal Compound
- Laparoscopic Extracorporporeal Knot Thying Using an Instrument for Knot Pushing and Tightening
- A New Technique for Single Spore Isolation of Two Predacious Fungi Forming Constricting Ring