Yonsei Med J.
2019 Feb;60(2):200-207. 10.3349/ymj.2019.60.2.200.
Signal Detection for Cardiovascular Adverse Events of DPP-4 Inhibitors Using the Korea Adverse Event Reporting System Database, 2008–2016
- Affiliations
-
- 1School of Pharmacy, Sungkyunkwan University, Suwon, Korea. shin.jy@skku.edu
- 2National Institute of Food and Drug Safety Evaluation, Korea Ministry of Food and Drug Safety, Cheongju, Korea.
- KMID: 2431637
- DOI: http://doi.org/10.3349/ymj.2019.60.2.200
Abstract
- PURPOSE
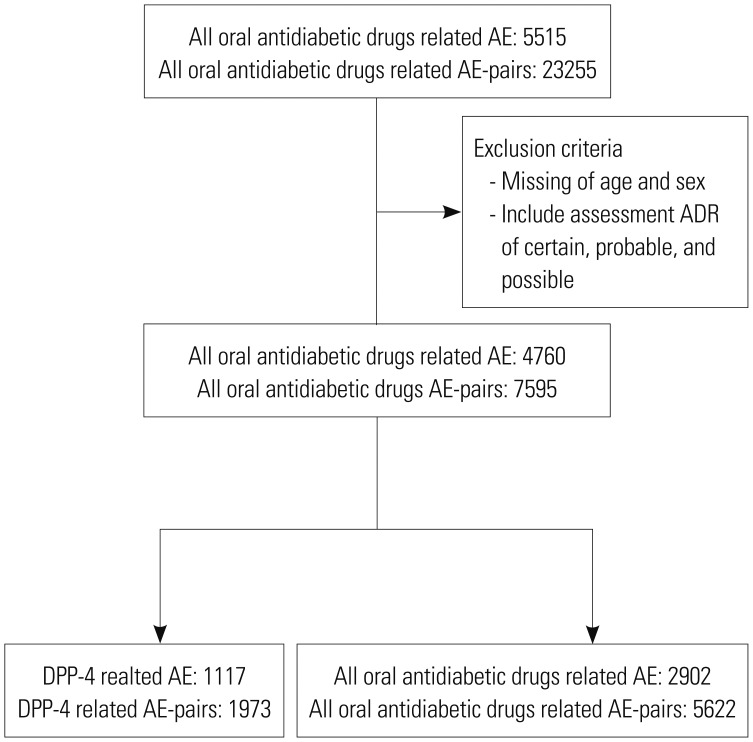
Cardiovascular adverse events (AEs) after use of dipeptidyl peptidase-4 (DPP4) inhibitors have been reported and suspected since the launch of DPP-4 inhibitors in 2006. However, few studies have investigated the association between cardiovascular AEs and DPP-4 inhibitors. The objective of this study is to detect the signals of cardiovascular AEs after use of DPP-4 inhibitors by analyzing the Korea Institute of Drug Safety & Risk Management-Korea Adverse Event Reporting System Database (KIDS-KD).
MATERIALS AND METHODS
Data on the use of oral antidiabetic drugs from 2008 to 2016 were extracted from KIDS-KD, and analyzed descriptively. Data mining was conducted by calculating three indices, which were proportional reporting ratios, reporting odds ratios, and information components, to detect signals from use of all oral antidiabetic drugs including DPP-4 inhibitors. Then, the suspected adverse drug reactions (ADRs) were confirmed by signal detection, and drug label information between the Korea Ministry of Food and Drug Safety and the U.S. Food and Drug Administration were compared.
RESULTS
Cardiovascular AEs after taking DPP-4 inhibitors were detected in only three (1.0%) out of a total of 307 AE reports. Two of the three cardiovascular AEs were reported after using sitagliptin and one using gemiglipitin, but these were not statistically significant.
CONCLUSION
Analysis of spontaneous ADR reports data on the use of DPP-4 inhibitors could not showed the association between DPP-4 inhibitors and cardiovascular AEs, due to a small number of cardiovascular AEs reports.
MeSH Terms
Figure
Reference
-
1. Lundkvist J, Jönsson B. Pharmacoeconomics of adverse drug reactions. Fundam Clin Pharmacol. 2004; 18:275–280. PMID: 15147278.
Article2. Kim MG, Kang HR, Kim JH, et al. Analysis of adverse drug reactions collected by an electronic reporting system in a single hospital. Korean J Med. 2009; 77:601–609.3. Choi NK, Park BJ. [Adverse drug reaction surveillance system in Korea]. J Prev Med Public Health. 2007; 40:278–284. PMID: 17693730.
Article4. Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet. 2000; 356:1255–1259. PMID: 11072960.
Article5. Hauben M, Madigan D, Gerrits CM, Walsh L, Van Puijenbroek EP. The role of data mining in pharmacovigilance. Expert Opin Drug Saf. 2005; 4:929–948. PMID: 16111454.
Article6. Uppsala Monitoring Centre. What is a signal? accessed on 2018 March 31. Available at: https://www.who-umc.org/research-scientific-development/signal-detection/what-is-a-signal/.7. Korea Centers for Disease Control & Prevention Call Center. Health behavior and chronic disease statistics: national health nutrition survey and youth health behavior online survey in 2015. Cheongju: Korea Centers for Disease Control;2016.8. Statistics Korea. Cause of death of Koreans in 2016. Daejeon: Statistics Korea;2017.9. Korean Diabetes Association. Treatment guideline for diabetes. Seoul: Korean Diabetes Association;2015.10. Udell JA, Cavender MA, Bhatt DL, Chatterjee S, Farkouh ME, Scirica BM. Glucose-lowering drugs or strategies and cardiovascular outcomes in patients with or at risk for type 2 diabetes: a meta-analysis of randomised controlled trials. Lancet Diabetes Endocrinol. 2015; 3:356–366. PMID: 25791290.
Article11. Wu S, Hopper I, Skiba M, Krum H. Dipeptidyl peptidase-4 inhibitors and cardiovascular outcomes: meta-analysis of randomized clinical trials with 55,141 participants. Cardiovasc Ther. 2014; 32:147–158. PMID: 24750644.
Article12. Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013; 369:1317–1326. PMID: 23992601.
Article13. Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015; 373:232–242. PMID: 26052984.
Article14. Weir DL, McAlister FA, Senthilselvan A, Minhas-Sandhu JK, Eurich DT. Sitagliptin use in patients with diabetes and heart failure: a population-based retrospective cohort study. JACC Heart Fail. 2014; 2:573–582. PMID: 24998080.15. Wang KL, Liu CJ, Chao TF, Huang CM, Wu CH, Chen SJ, et al. Sitagliptin and the risk of hospitalization for heart failure: a population-based study. Int J Cardiol. 2014; 177:86–90. PMID: 25499347.
Article16. Savarese G, Perrone-Filardi P, D'Amore C, Vitale C, Trimarco B, Pani L, et al. Cardiovascular effects of dipeptidyl peptidase-4 inhibitors in diabetic patients: a meta-analysis. Int J Cardiol. 2015; 181:239–244. PMID: 25528528.
Article17. White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013; 369:1327–1335. PMID: 23992602.
Article18. Korea Institute of Drug Safety & Risk Management. Manual for Korea Institute of Drug Safety & Risk Management Korea Adverse Event Reporting System Database, KIDS-KD. accessed on 2018 March 31. Available at: https://www.drugsafe.or.kr/cmm/fms/FileDown.do;jsessionid=p6gBKyqO0PXEHacyyWWDjoepePOMhmgYlbvG2fdzi1Cj7Tj4QvnkWSxyGveaLFOd.webint_2_servlet_engine1?atchFileId=FILE_000000000036473&fileSn=1&flage=0.19. Korea Institute of Drug Safety & Risk Management. Reporting adverse drug reactions; definitions of terms and criteria for their use. accessed on 2018 March 31. Available at: https://www.drugsafe.or.kr/cmm/fms/FileDown.do;jsessionid=57QMs9trcwgvYD4jaSx6N0EWWBBmkD8UJnGM8RzFCBEwaBWl9Yal7sCv9r6p6jNn.webint_2_servlet_engine1?atchFileId=FILE_000000000035522&fileSn=0&flage=0.20. Poluzzi E, Raschi E, Piccinni C, De Ponti F. Data mining techniques in pharmacovigilance: analysis of the publicly accessible FDA adverse event reporting system (AERS). In : Karahoca A, editor. Data mining applications in engineering and medicine. London: IntechOpen;2012. p. 265–302.21. Bate A, Pariente A, Hauben M, Bégaud B. Quantitative signal detection and analysis in pharmacovigilance. In : Andrews EB, Moore N, editors. Mann's pharmacovigilance. 3rd ed. New York: Wiley Blackwell;2014. p. 331–354.22. Miller RR. Hospital admissions due to adverse drug reactions. A report from the Boston Collaborative Drug Surveillance Program. Arch Intern Med. 1974; 134:219–223. PMID: 4843186.
Article23. Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998; 279:1200–1205. PMID: 9555760.
Article24. Raschi E, Poluzzi E, Koci A, Antonazzo IC, Marchesini G, De Ponti F. Dipeptidyl peptidase-4 inhibitors and heart failure: analysis of spontaneous reports submitted to the FDA Adverse Event Reporting System. Nutr Metab Cardiovasc Dis. 2016; 26:380–386. PMID: 27067162.
Article25. Kim MS, Woo YJ, Shin SM, Kim JY, Jung SY, Park BJ. Signal detection and safety information generation of aripiprazole in spontaneous adverse event reports database. Korean J Psychopharmacol. 2015; 26:10–16.26. Ministry of Food and Drug Safety White Paper. Osong: Ministry of Food and Drug Safety;2016.27. Ahn SH, Chung S, Jung SY, Shin JY, Park BJ. Awareness of adverse drug reaction reporting system in general population. Health Policy Manag. 2014; 24:164–171.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Signal Detection of DPP-IV Inhibitors using Spontaneous Adverse Event Reporting System in Korea
- A Class-Effect Study of Vaccine Signal Detection Using Korea Adverse Event Reporting System Database
- Signal Detection for Adverse Events of Finasteride Using Korea Adverse Event Reporting System (KAERS) Database
- Analysis of Important Medical Adverse Events and Signals Related with Cyclosporine and Tacrolimus Using the FDA Adverse Event Reporting System (FAERS) Database
- Signal Detection and Safety Information Generation of Aripiprazole in Spontaneous Adverse Event Reports Database