Korean J Radiol.
2019 Jan;20(1):5-17. 10.3348/kjr.2018.0040.
What Is New in the 2017 World Health Organization Classification and 8th American Joint Committee on Cancer Staging System for Pancreatic Neuroendocrine Neoplasms?
- Affiliations
-
- 1Department of Radiology and Research Institute of Radiology, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea. medimash@gmail.com
- 2Department of Oncology, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea.
- 3Department of Pathology, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea.
- 4Department of Nuclear Medicine, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea.
- 5Department of Imaging, Dana-Farber Cancer Institute, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, USA.
- 6Department of Imaging, UH Cleveland Medical Center, Case Western Reserve University, Cleveland, OH, USA.
- KMID: 2429916
- DOI: http://doi.org/10.3348/kjr.2018.0040
Abstract
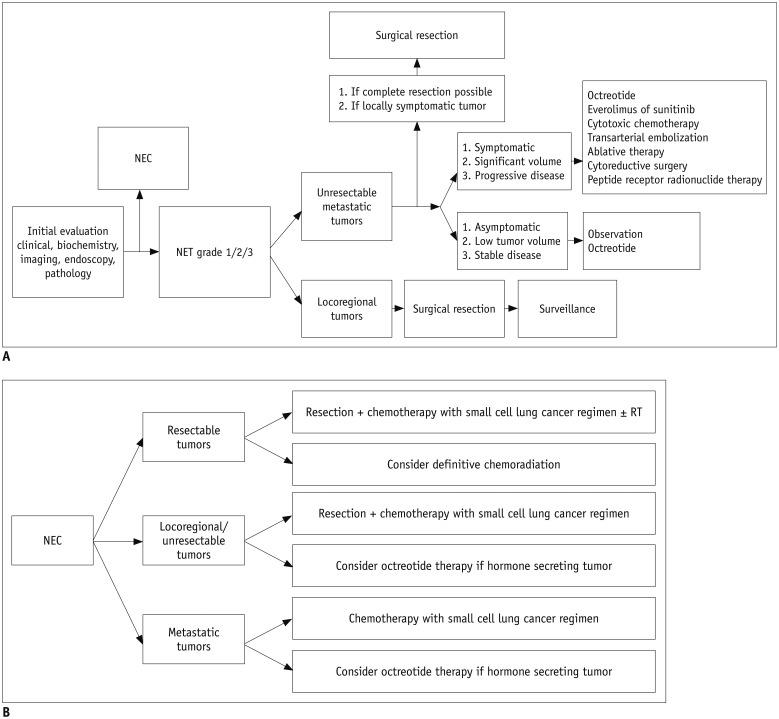
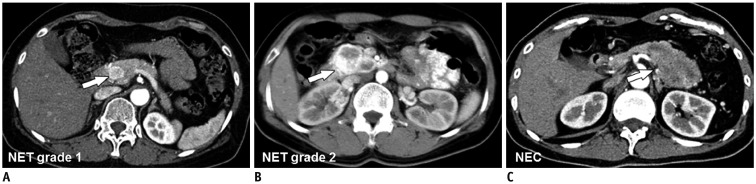
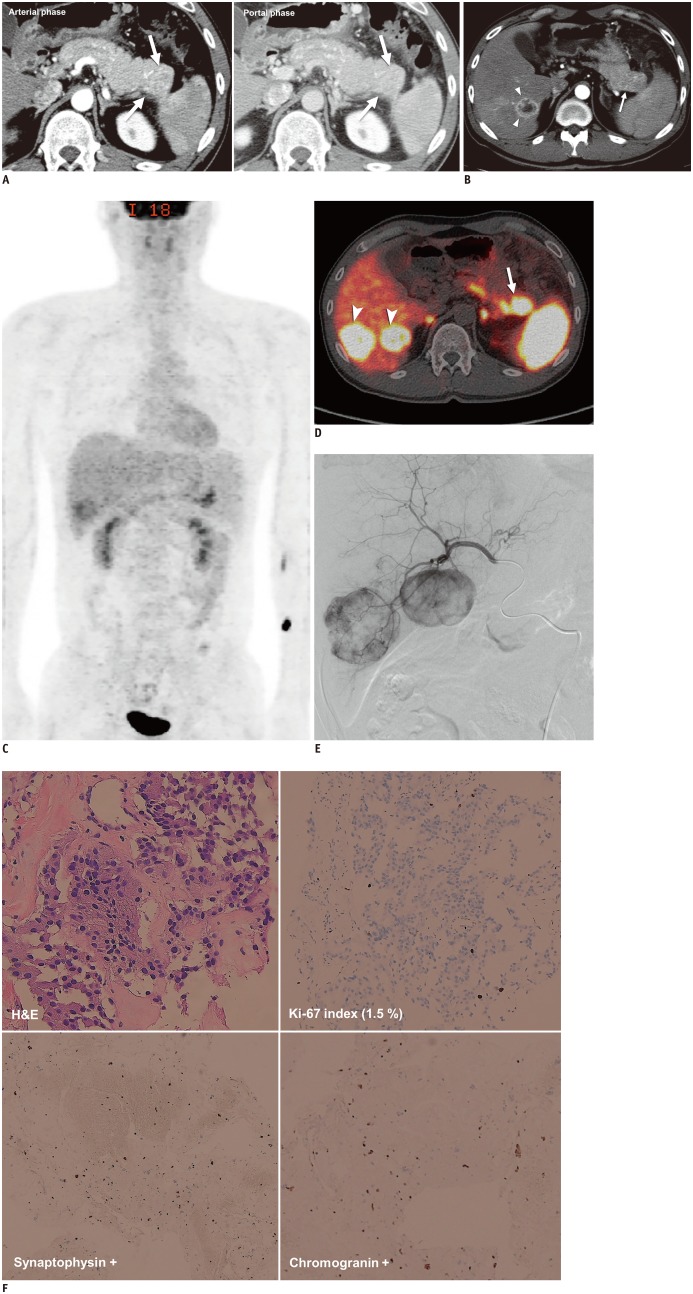
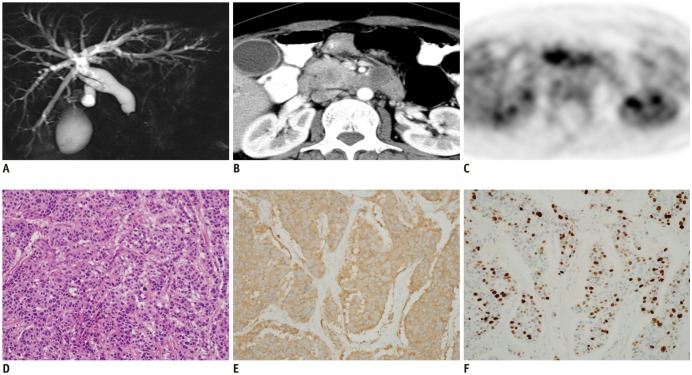
- The diagnosis and management of pancreatic neuroendocrine neoplasms (NENs) have evolved significantly in recent years. There are several diagnostic and therapeutic challenges and controversies regarding the management of these lesions. In this review, we focus on the recent significant changes and controversial issues regarding the diagnosis and management of NENs and discuss the role of imaging in the multidisciplinary team approach.
Keyword
MeSH Terms
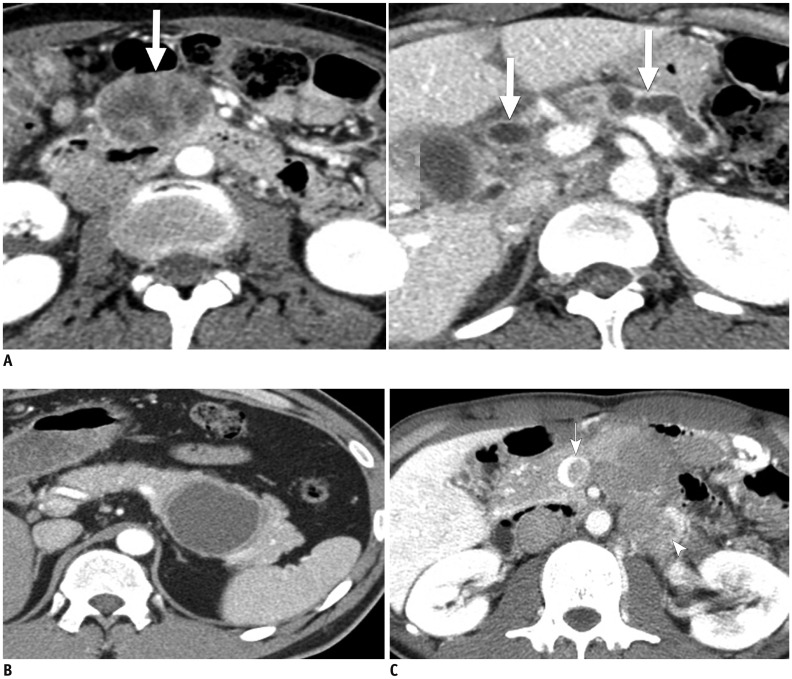
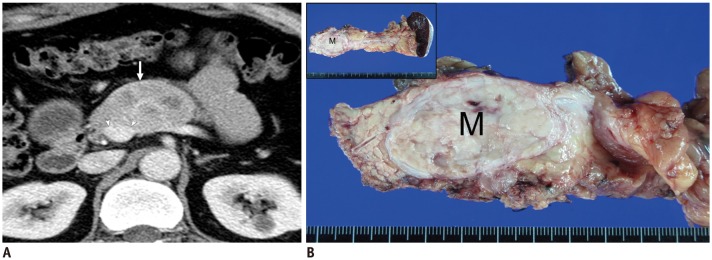
Figure
Reference
-
1. Singhi AD, Klimstra DS. Well-differentiated pancreatic neuroendocrine tumours (PanNETs) and poorly differentiated pancreatic neuroendocrine carcinomas (PanNECs): concepts, issues and a practical diagnostic approach to high-grade (G3) cases. Histopathology. 2018; 72:168–177. PMID: 29239037.
Article2. Lawrence B, Gustafsson BI, Chan A, Svejda B, Kidd M, Modlin IM. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am. 2011; 40:1–18. PMID: 21349409.
Article3. Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system. 4th ed. Lyon: International Agency for Research on Cancer;2010.4. Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas. 2010; 39:707–712. PMID: 20664470.5. Edge S, Byrd DR, Compton CC, Fritz AG, Greene F, Trotti A. AJCC cancer staging manual. 7th ed. New York, NY: Springer;2010. p. 181–190.6. Rindi G, Klöppel G, Alhman H, Caplin M, Couvelard A, de Herder WW, et al. all other Frascati Consensus Conference participants. European Neuroendocrine Tumor Society (ENETS). TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 2006; 449:395–401. PMID: 16967267.
Article7. Klimstra DS, Modlin IR, Adsay NV, Chetty R, Deshpande V, Gönen M, et al. Pathology reporting of neuroendocrine tumors: application of the Delphic consensus process to the development of a minimum pathology data set. Am J Surg Pathol. 2010; 34:300–313. PMID: 20118772.
Article8. Klöppel G, Couvelard A, Perren A, Komminoth P, McNicol AM, Nilsson O, et al. Mallorca Consensus Conference participants. European Neuroendocrine Tumor Society. ENETS consensus guidelines for the standards of care in neuroendocrine tumors: towards a standardized approach to the diagnosis of gastroenteropancreatic neuroendocrine tumors and their prognostic stratification. Neuroendocrinology. 2009; 90:162–166. PMID: 19060454.
Article9. NCCN clinical practice guidelines in oncology. Neuroendocrine tumors. Version 3. National Comprehensive Cancer Network Web site. Accessed December 16, 2017. https://www.nccn.org/professionals/physician_gls/pdf/neuroendocrine.pdf Published June 13, 2017.10. Lloyd RV, Osamura RY, Klöppel G, Rosai J. WHO classification of tumours of endocrine organs. 4th ed. Lyon: International Agency for Research on Cancer;2017. p. 209–240.11. Basturk O, Yang Z, Tang LH, Hruban RH, Adsay V, McCall CM, et al. The high-grade (WHO G3) pancreatic neuroendocrine tumor category is morphologically and biologically heterogenous and includes both well differentiated and poorly differentiated neoplasms. Am J Surg Pathol. 2015; 39:683–690. PMID: 25723112.
Article12. Adsay NV, Bagci P, Tajiri T, Oliva I, Ohike N, Balci S, et al. Pathologic staging of pancreatic, ampullary, biliary, and gallbladder cancers: pitfalls and practical limitations of the current AJCC/UICC TNM staging system and opportunities for improvement. Semin Diagn Pathol. 2012; 29:127–141. PMID: 23062420.
Article13. Rindi G, Falconi M, Klersy C, Albarello L, Boninsegna L, Buchler MW, et al. TNM staging of neoplasms of the endocrine pancreas: results from a large international cohort study. J Natl Cancer Inst. 2012; 104:764–777. PMID: 22525418.
Article14. Li X, Gou S, Liu Z, Ye Z, Wang C. Assessment of the American Joint Commission on Cancer 8th Edition Staging System for patients with pancreatic neuroendocrine tumors: a surveillance, epidemiology, and end results analysis. Cancer Med. 2018; 7:626–634. PMID: 29380547.
Article15. Luo G, Javed A, Strosberg JR, Jin K, Zhang Y, Liu C, et al. Modified staging classification for pancreatic neuroendocrine tumors on the basis of the American Joint Committee on Cancer and European Neuroendocrine Tumor Society systems. J Clin Oncol. 2017; 35:274–280. PMID: 27646952.
Article16. Bushnell DL Jr, O'Dorisio TM, O'Dorisio MS, Menda Y, Hicks RJ, Van Cutsem E, et al. 90Y-edotreotide for metastatic carcinoid refractory to octreotide. J Clin Oncol. 2010; 28:1652–1659. PMID: 20194865.
Article17. Ramage JK, Ahmed A, Ardill J, Bax N, Breen DJ, Caplin ME, et al. UK and Ireland Neuroendocrine Tumour Society. Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours (NETs). Gut. 2012; 61:6–32. PMID: 22052063.
Article18. Reid MD, Balci S, Saka B, Adsay NV. Neuroendocrine tumors of the pancreas: current concepts and controversies. Endocr Pathol. 2014; 25:65–79. PMID: 24430597.
Article19. Raj N, Valentino E, Capanu M, Tang LH, Basturk O, Untch BR, et al. Treatment response and outcomes of grade 3 pancreatic neuroendocrine neoplasms based on morphology: well differentiated versus poorly differentiated. Pancreas. 2017; 46:296–301. PMID: 27759713.20. Burns WR, Edil BH. Neuroendocrine pancreatic tumors: guidelines for management and update. Curr Treat Options Oncol. 2012; 13:24–34. PMID: 22198808.
Article21. Parekh JR, Wang SC, Bergsland EK, Venook AP, Warren RS, Kim GE, et al. Lymph node sampling rates and predictors of nodal metastasis in pancreatic neuroendocrine tumor resections: the UCSF experience with 149 patients. Pancreas. 2012; 41:840–844. PMID: 22781907.22. Hill JS, McPhee JT, McDade TP, Zhou Z, Sullivan ME, Whalen GF, et al. Pancreatic neuroendocrine tumors: the impact of surgical resection on survival. Cancer. 2009; 115:741–751. PMID: 19130464.23. Kim KW, Krajewski KM, Nishino M, Jagannathan JP, Shinagare AB, Tirumani SH, et al. Update on the management of gastroenteropancreatic neuroendocrine tumors with emphasis on the role of imaging. AJR Am J Roentgenol. 2013; 201:811–824. PMID: 24059370.
Article24. Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, et al. NETTER-1 Trial Investigators. Phase 3 trial of 177Lu-dotatate for midgut neuroendocrine tumors. N Engl J Med. 2017; 376:125–135. PMID: 28076709.25. Villard L, Romer A, Marincek N, Brunner P, Koller MT, Schindler C, et al. Cohort study of somatostatin-based radiopeptide therapy with [(90)Y-DOTA]-TOC versus [(90)Y-DOTA]-TOC plus [(177)Lu-DOTA]-TOC in neuroendocrine cancers. J Clin Oncol. 2012; 30:1100–1106. PMID: 22393097.
Article26. Terashima T, Morizane C, Hiraoka N, Tsuda H, Tamura T, Shimada Y, et al. Comparison of chemotherapeutic treatment outcomes of advanced extrapulmonary neuroendocrine carcinomas and advanced small-cell lung carcinoma. Neuroendocrinology. 2012; 96:324–332. PMID: 22572060.
Article27. Sundstrøm S, Bremnes RM, Kaasa S, Aasebø U, Hatlevoll R, Dahle R, et al. Norwegian Lung Cancer Study Group. Cisplatin and etoposide regimen is superior to cyclophosphamide, epirubicin, and vincristine regimen in small-cell lung cancer: results from a randomized phase III trial with 5 years' follow-up. J Clin Oncol. 2002; 20:4665–4672. PMID: 12488411.
Article28. Kim DW, Kim HJ, Kim KW, Byun JH, Song KB, Kim JH, et al. Neuroendocrine neoplasms of the pancreas at dynamic enhanced CT: comparison between grade 3 neuroendocrine carcinoma and grade 1/2 neuroendocrine tumour. Eur Radiol. 2015; 25:1375–1383. PMID: 25465713.
Article29. Takumi K, Fukukura Y, Higashi M, Ideue J, Umanodan T, Hakamada H, et al. Pancreatic neuroendocrine tumors: correlation between the contrast-enhanced computed tomography features and the pathological tumor grade. Eur J Radiol. 2015; 84:1436–1443. PMID: 26022520.
Article30. Kim C, Byun JH, Hong SM, An S, Kim JH, Lee SS, et al. A comparison of enhancement patterns on dynamic enhanced CT and survival between patients with pancreatic neuroendocrine tumors with and without intratumoral fibrosis. Abdom Radiol (NY). 2017; 42:2835–2842. PMID: 28624923.
Article31. Cappelli C, Boggi U, Mazzeo S, Cervelli R, Campani D, Funel N, et al. Contrast enhancement pattern on multidetector CT predicts malignancy in pancreatic endocrine tumours. Eur Radiol. 2015; 25:751–759. PMID: 25447971.
Article32. Rust E, Hubele F, Marzano E, Goichot B, Pessaux P, Kurtz JE, et al. Nuclear medicine imaging of gastro-entero-pancreatic neuroendocrine tumors. The key role of cellular differentiation and tumor grade: from theory to clinical practice. Cancer Imaging. 2012; 12:173–184. PMID: 22743056.
Article33. Lewis RB, Lattin GE Jr, Paal E. Pancreatic endocrine tumors: radiologic-clinicopathologic correlation. Radiographics. 2010; 30:1445–1464. PMID: 21071369.
Article34. Gabriel M, Decristoforo C, Kendler D, Dobrozemsky G, Heute D, Uprimny C, et al. 68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: comparison with somatostatin receptor scintigraphy and CT. J Nucl Med. 2007; 48:508–518. PMID: 17401086.
Article35. Haug AR, Auernhammer CJ, Wängler B, Schmidt GP, Uebleis C, Göke B, et al. 68Ga-DOTATATE PET/CT for the early prediction of response to somatostatin receptor-mediated radionuclide therapy in patients with well-differentiated neuroendocrine tumors. J Nucl Med. 2010; 51:1349–1356. PMID: 20720050.
Article36. Sharma P, Singh H, Bal C, Kumar R. PET/CT imaging of neuroendocrine tumors with (68)Gallium-labeled somatostatin analogues: an overview and single institutional experience from India. Indian J Nucl Med. 2014; 29:2–12. PMID: 24591775.37. Gallotti A, Johnston RP, Bonaffini PA, Ingkakul T, Deshpande V, Fernández-del Castillo C, et al. Incidental neuroendocrine tumors of the pancreas: MDCT findings and features of malignancy. AJR Am J Roentgenol. 2013; 200:355–362. PMID: 23345357.
Article38. Singhi AD, Chu LC, Tatsas AD, Shi C, Ellison TA, Fishman EK, et al. Cystic pancreatic neuroendocrine tumors: a clinicopathologic study. Am J Surg Pathol. 2012; 36:1666–1673. PMID: 23073325.39. Lee JH, Byun JH, Kim JH, Lee SS, Kim HJ, Lee MG. Solid pancreatic tumors with unilocular cyst-like appearance on CT: differentiation from unilocular cystic tumors using CT. Korean J Radiol. 2014; 15:704–711. PMID: 25469081.
Article40. Shi C, Siegelman SS, Kawamoto S, Wolfgang CL, Schulick RD, Maitra A, et al. Pancreatic duct stenosis secondary to small endocrine neoplasms: a manifestation of serotonin production? Radiology. 2010; 257:107–114. PMID: 20713615.
Article41. Lam KY, Lo CY. Pancreatic endocrine tumour: a 22-year clinico-pathological experience with morphological, immunohistochemical observation and a review of the literature. Eur J Surg Oncol. 1997; 23:36–42. PMID: 9066745.
Article42. Volante M, Righi L, Berruti A, Rindi G, Papotti M. The pathological diagnosis of neuroendocrine tumors: common questions and tentative answers. Virchows Arch. 2011; 458:393–402. PMID: 21344263.
Article43. Balachandran A, Tamm EP, Bhosale PR, Katz MH, Fleming JB, Yao JC, et al. Venous tumor thrombus in nonfunctional pancreatic neuroendocrine tumors. AJR Am J Roentgenol. 2012; 199:602–608. PMID: 22915400.
Article44. Kulke MH, Siu LL, Tepper JE, Fisher G, Jaffe D, Haller DG, et al. Future directions in the treatment of neuroendocrine tumors: consensus report of the National Cancer Institute neuroendocrine tumor clinical trials planning meeting. J Clin Oncol. 2011; 29:934–943. PMID: 21263089.
Article45. Oberg K, Casanovas O, Castaño JP, Chung D, Delle Fave G, Denèfle P, et al. Molecular pathogenesis of neuroendocrine tumors: implications for current and future therapeutic approaches. Clin Cancer Res. 2013; 19:2842–2849. PMID: 23459719.
Article46. Kim JH, Lee JM, Park JH, Kim SC, Joo I, Han JK, et al. Solid pancreatic lesions: characterization by using timing bolus dynamic contrast-enhanced MR imaging assessment--a preliminary study. Radiology. 2013; 266:185–196. PMID: 23192779.
Article47. Bali MA, Metens T, Denolin V, Delhaye M, Demetter P, Closset J, et al. Tumoral and nontumoral pancreas: correlation between quantitative dynamic contrast-enhanced MR imaging and histopathologic parameters. Radiology. 2011; 261:456–466. PMID: 21852570.
Article48. Hwang EJ, Lee JM, Yoon JH, Kim JH, Han JK, Choi BI, et al. Intravoxel incoherent motion diffusion-weighted imaging of pancreatic neuroendocrine tumors: prediction of the histologic grade using pure diffusion coefficient and tumor size. Invest Radiol. 2014; 49:396–402. PMID: 24500090.49. Koh DM, Collins DJ, Orton MR. Intravoxel incoherent motion in body diffusion-weighted MRI: reality and challenges. AJR Am J Roentgenol. 2011; 196:1351–1361. PMID: 21606299.
Article50. De Cecco CN, Darnell A, Rengo M, Muscogiuri G, Bellini D, Ayuso C, et al. Dual-energy CT: oncologic applications. AJR Am J Roentgenol. 2012; 199(5 Suppl):S98–S105. PMID: 23097174.
Article51. Kartalis N, Mucelli RM, Sundin A. Recent developments in imaging of pancreatic neuroendocrine tumors. Ann Gastroenterol. 2015; 28:193–202. PMID: 25830417.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recent Update of Pathology of the Pancreatic Neuroendocrine Tumor
- Assessment of the Prognostic Staging System of American Joint Committee on Cancer 8th Edition for Breast Cancer: Comparisons with the Conventional Anatomic Staging System
- Comparison of the Differences in Survival Rates between the 7th and 8th Editions of the AJCC TNM Staging System for Gastric Adenocarcinoma: a Single-Institution Study of 5,507 Patients in Korea
- Introduction of 7th AJCC TNM Staging for Hepatobiliary, Pancreatic Ampulla of Vater, Exocrine and Endocrine Cancers
- Updated guidelines on the preoperative staging of thyroid cancer