J Gastric Cancer.
2018 Dec;18(4):356-367. 10.5230/jgc.2018.18.e35.
KLK6 Promotes Growth, Migration, and Invasion of Gastric Cancer Cells
- Affiliations
-
- 1Department of Surgery, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China. shengxing_zhu@sina.com
- 2Department of The Second General Surgery, People's Hospital of Zhengzhou, Zhengzhou, China.
- 3School of Basic Medicine, Zhengzhou University, Zhengzhou, China.
- 4Department of Gastrointestinal Surgery, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China.
- KMID: 2429882
- DOI: http://doi.org/10.5230/jgc.2018.18.e35
Abstract
- PURPOSE
Kallikrein (KLK) proteases are hormone-like signaling molecules with critical functions in different cancers. This study investigated the expression of KLK6 in gastric cancer and its potential role in the growth, migration, and invasion of gastric cancer cells.
MATERIALS AND METHODS
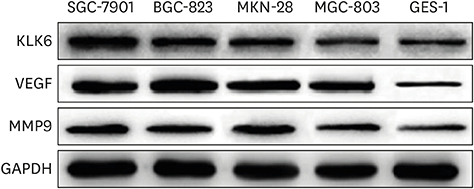
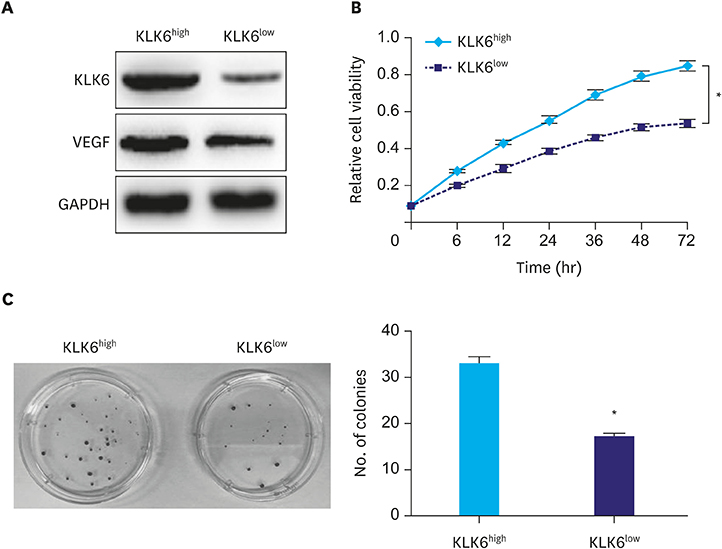
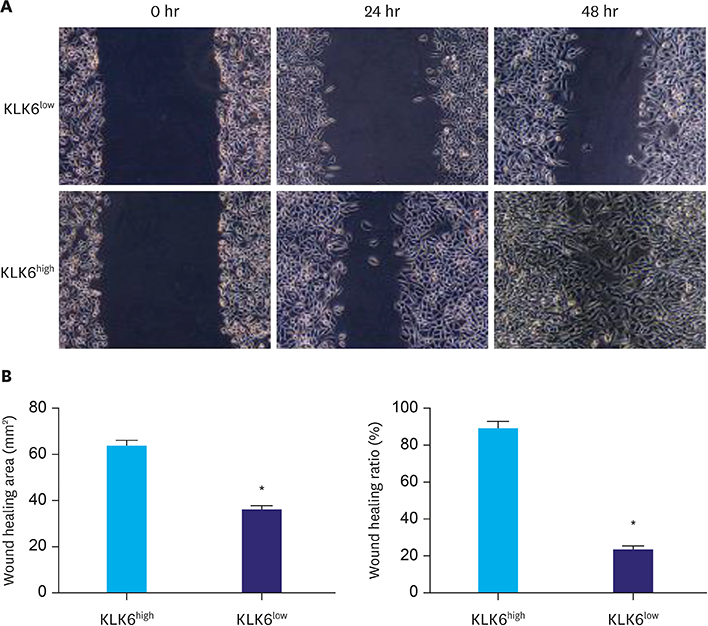
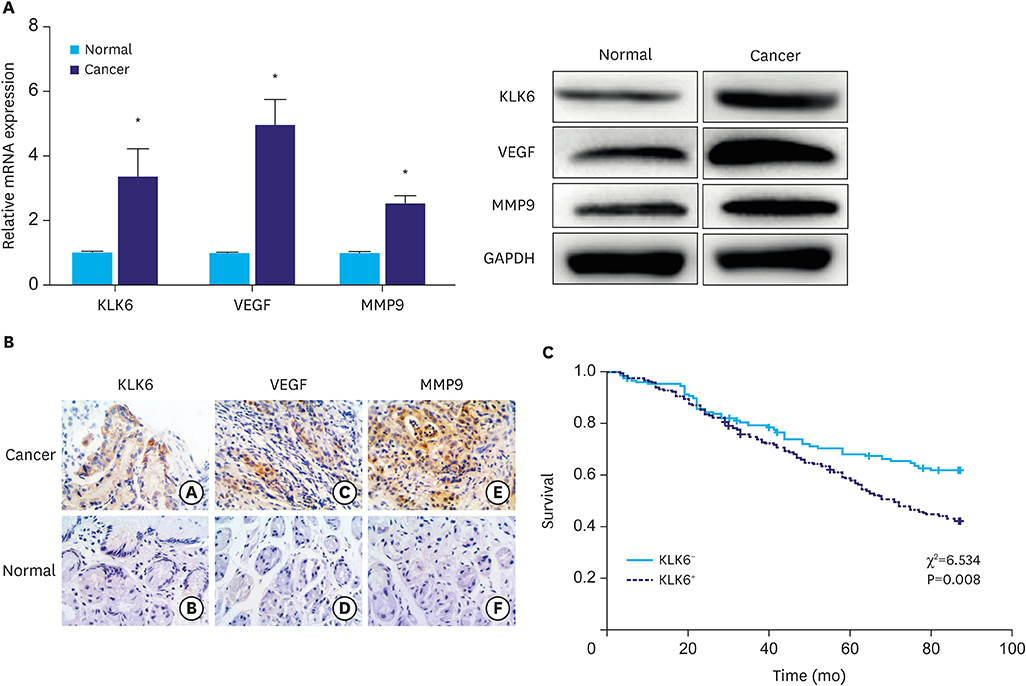
In this study, we compared protein levels of KLK6, vascular endothelial growth factor (VEGF), and matrix metallopeptidase (MMP) 9 in normal gastric epithelial and gastric cancer cell lines by western blot. Fluorescence-activated cell sorting was employed to sort 2 clones of SGC-7901 cells with distinct KLK6 expression, namely, KLK6-high (KLK6high) and KLK6-low (KLK6low), which were then expanded. Lastly, immunohistochemical analysis was performed to investigate KLK6 expression in gastric cancer patients.
RESULTS
The expression levels of KLK6, VEGF, and MMP 9, were significantly higher in the gastric cancer cell lines SGC-7901, BGC-823, MKN-28, and MGC-803 than in the normal gastric epithelial cell line GES-1. Compared to KLK6low cells, KLK6high cells showed enhanced viability, colony-forming ability, migration, and invasion potential in vitro. Importantly, immunohistochemical analysis of a human gastric cancer tissue cohort revealed that the staining for KLK6, VEGF, and MMP9 was markedly stronger in the cancerous tissues than in the adjacent normal tissues. KLK6 expression also correlated with that of VEGF and MMP9 expression, as well as several key clinicopathological parameters.
CONCLUSIONS
Together, these results suggest an important role for KLK6 in human gastric cancer progression.
Keyword
MeSH Terms
Figure
Reference
-
1. Yousef GM, Diamandis EP. The new human tissue kallikrein gene family: structure, function, and association to disease. Endocr Rev. 2001; 22:184–204.
Article2. Borgoño CA, Diamandis EP. The emerging roles of human tissue kallikreins in cancer. Nat Rev Cancer. 2004; 4:876–890.
Article3. Jin Y, Qu S, Tesikova M, Wang L, Kristian A, Mælandsmo GM, et al. Molecular circuit involving KLK4 integrates androgen and mTOR signaling in prostate cancer. Proc Natl Acad Sci U S A. 2013; 110:E2572–E2581.
Article4. Stamey TA, Yang N, Hay AR, McNeal JE, Freiha FS, Redwine E. Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N Engl J Med. 1987; 317:909–916.
Article5. Nagahara H, Mimori K, Utsunomiya T, Barnard GF, Ohira M, Hirakawa K, et al. Clinicopathologic and biological significance of kallikrein 6 overexpression in human gastric cancer. Clin Cancer Res. 2005; 11:6800–6806.
Article6. Schuster R, Max N, Mann B, Heufelder K, Thilo F, Gröne J, et al. Quantitative real-time RT-PCR for detection of disseminated tumor cells in peripheral blood of patients with colorectal cancer using different mRNA markers. Int J Cancer. 2004; 108:219–227.
Article7. Nathalie HV, Chris P, Serge G, Catherine C, Benjamin B, Claire B, et al. High kallikrein-related peptidase 6 in non-small cell lung cancer cells: an indicator of tumour proliferation and poor prognosis. J Cell Mol Med. 2009; 13:9B. 4014–4022.
Article8. Shan SJ, Scorilas A, Katsaros D, Diamandis EP. Transcriptional upregulation of human tissue kallikrein 6 in ovarian cancer: clinical and mechanistic aspects. Br J Cancer. 2007; 96:362–372.
Article9. Vakrakou A, Devetzi M, Papachristopoulou G, Malachias A, Scorilas A, Xynopoulos D, et al. Kallikrein-related peptidase 6 (KLK6) expression in the progression of colon adenoma to carcinoma. Biol Chem. 2014; 395:1105–1117.10. Klucky B, Mueller R, Vogt I, Teurich S, Hartenstein B, Breuhahn K, et al. Kallikrein 6 induces E-cadherin shedding and promotes cell proliferation, migration, and invasion. Cancer Res. 2007; 67:8198–8206.
Article11. Kim JT, Song EY, Chung KS, Kang MA, Kim JW, Kim SJ, et al. Up-regulation and clinical significance of serine protease kallikrein 6 in colon cancer. Cancer. 2011; 117:2608–2619.
Article12. Sawada K, Mitra AK, Radjabi AR, Bhaskar V, Kistner EO, Tretiakova M, et al. Loss of E-cadherin promotes ovarian cancer metastasis via alpha 5-integrin, which is a therapeutic target. Cancer Res. 2008; 68:2329–2339.13. Kim TW, Lee SJ, Kim JT, Kim SJ, Min JK, Bae KH, et al. Kallikrein-related peptidase 6 induces chemotherapeutic resistance by attenuating auranofin-induced cell death through activation of autophagy in gastric cancer. Oncotarget. 2016; 7:85332–85348.
Article14. Masoumi Moghaddam S, Amini A, Morris DL, Pourgholami MH. Significance of vascular endothelial growth factor in growth and peritoneal dissemination of ovarian cancer. Cancer Metastasis Rev. 2012; 31:143–162.
Article15. Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006; 25:9–34.
Article16. Carcas LP. Gastric cancer review. J Carcinog. 2014; 13:14.
Article17. Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016; 388:2654–2664.
Article18. Lenga Ma Bonda W, Iochmann S, Magnen M, Courty Y, Reverdiau P. Kallikrein-related peptidases in lung diseases. Biol Chem. 2018; 399:959–971.
Article19. Kim JJ, Kim JT, Yoon HR, Kang MA, Kim JH, Lee YH, et al. Upregulation and secretion of kallikrein-related peptidase 6 (KLK6) in gastric cancer. Tumour Biol. 2012; 33:731–738.20. Oikonomopoulou K, Diamandis EP, Hollenberg MD. Kallikrein-related peptidases: proteolysis and signaling in cancer, the new frontier. Biol Chem. 2010; 391:299–310.
Article21. Sananes A, Cohen I, Shahar A, Hockla A, De Vita E, Miller AK, et al. A potent, proteolysis-resistant inhibitor of kallikrein-related peptidase 6 (KLK6) for cancer therapy, developed by combinatorial engineering. J Biol Chem. 2018; 293:12663–12680.22. Sidiropoulos KG, Ding Q, Pampalakis G, White NM, Boulos P, Sotiropoulou G, et al. KLK6-regulated miRNA networks activate oncogenic pathways in breast cancer subtypes. Mol Oncol. 2016; 10:993–1007.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- LETM1 Promotes Gastric Cancer Cell Proliferation, Migration, and Invasion via the PI3K/Akt Signaling Pathway
- Circular RNA hsa_circ_0005556 Accelerates Gastric Cancer Progression by Sponging miR-4270 to Increase MMP19 Expression
- Enhancement of Cell Migration by Corticotropin-Releasing Hormone (CRH) in Human Gastric Cancer Cell Line, MKN-28
- Heat Shock Factor 1 Predicts Poor Prognosis of Gastric Cancer
- Beta-Catenin Downregulation Contributes to Epidermal Growth Factor-induced Migration and Invasion of MDAMB231 Cells