J Periodontal Implant Sci.
2018 Dec;48(6):337-346. 10.5051/jpis.2018.48.6.337.
Accelerated inflammation in peripheral artery disease patients with periodontitis
- Affiliations
-
- 1Department of Periodontology, Tokyo Medical and Dental University, Tokyo, Japan.
- 2Division of Periodontology, Department of Oral Interdisciplinary Medicine, Kanagawa Dental University Graduate School of Dentistry, Yokosuka, Japan. aoyama@kdu.ac.jp
- KMID: 2429862
- DOI: http://doi.org/10.5051/jpis.2018.48.6.337
Abstract
- PURPOSE
Peripheral artery disease (PAD) is a form of arteriosclerosis that occurs in the extremities and involves ischemia. Previous studies have reported that patients with periodontitis are at high risk for PAD. However, the relationship between these 2 diseases has not yet been fully elucidated. In this cross-sectional study, we investigated this relationship by comparing patients with PAD to those with arrhythmia (ARR) as a control group.
METHODS
A large-scale survey was conducted of patients with cardiovascular disease who visited Tokyo Medical and Dental University Hospital. We investigated their oral condition and dental clinical measurements, including probing pocket depth, bleeding on probing, clinical attachment level, and number of missing teeth; we also collected salivary and subgingival plaque samples and peripheral blood samples. All patients with PAD were extracted from the whole population (n = 25), and a matching number of patients with ARR were extracted (n = 25). Simultaneously, ARR patients were matched to PAD patients in terms of age, gender, prevalence of diabetes, hypertension, dyslipidemia, obesity, and the smoking rate (n = 25 in both groups). Real-time polymerase chain reaction was performed to measure the bacterial counts, while the enzyme-linked immunosorbent assay method was used to measure anti-bacterial antibody titers and proinflammatory cytokine levels in serum.
RESULTS
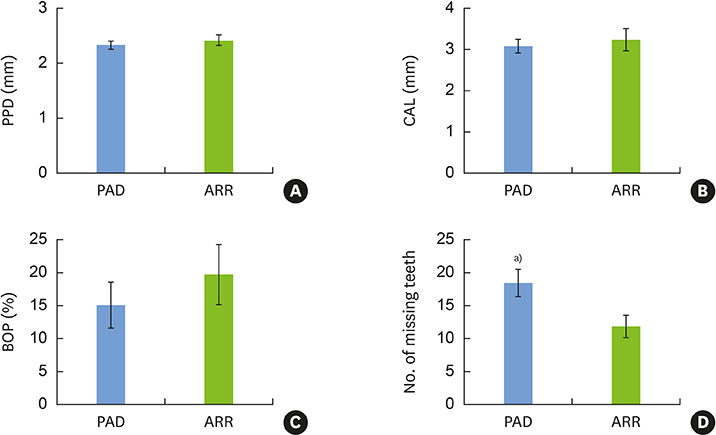
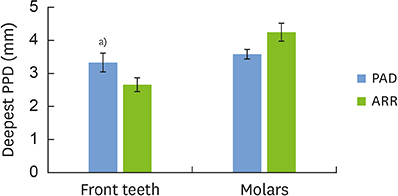
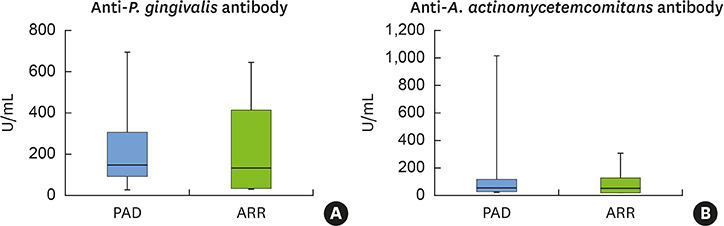
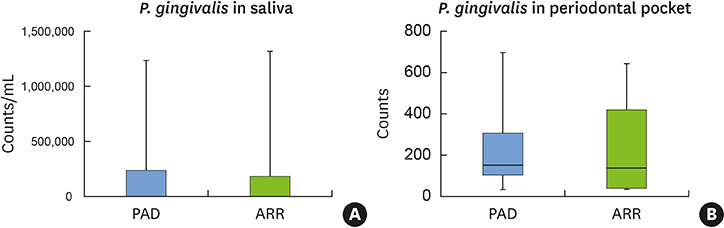
PAD patients had more missing teeth (18.4±2.0) and higher serum levels of C-reactive protein (1.57±0.85 mg/dL) and tumor necrosis factor-alpha (70.3±5.7 pg/mL) than ARR patients (12.0±1.7, 0.38±0.21 mg/dL, and 39.3±4.5 pg/mL, respectively). Meanwhile, no statistically significant differences were found in other dental clinical measurements, bacterial antibody titers, or bacterial counts between the 2 groups.
CONCLUSIONS
Our findings suggested that PAD patients had poorer oral and periodontal state with enhanced systemic inflammation.
MeSH Terms
-
Arrhythmias, Cardiac
Arteriosclerosis
Bacterial Load
C-Reactive Protein
Cardiovascular Diseases
Cross-Sectional Studies
Dyslipidemias
Enzyme-Linked Immunosorbent Assay
Extremities
Hemorrhage
Humans
Hypertension
Inflammation*
Ischemia
Methods
Obesity
Periodontitis*
Peripheral Arterial Disease*
Prevalence
Real-Time Polymerase Chain Reaction
Smoke
Smoking
Tooth
Tumor Necrosis Factor-alpha
C-Reactive Protein
Smoke
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Natsuaki C, Inoguchi T, Maeda Y, Yamada T, Sasaki S, Sonoda N, et al. Association of borderline ankle-brachial index with mortality and the incidence of peripheral artery disease in diabetic patients. Atherosclerosis. 2014; 234:360–365.
Article2. Higashi Y, Miyata T, Shigematsu H, Origasa H, Fujita M, Matsuo H, et al. Two-year follow-up of vascular events in peripheral arterial disease treated with antiplatelet agents: a prospective observational multicenter cohort study (SEASON). Sci Rep. 2017; 7:6095.
Article3. Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013; 382:1329–1340.
Article4. Tonetti MS, Greenwell H, Kornman KS. Staging and grading of periodontitis: framework and proposal of a new classification and case definition. J Clin Periodontol. 2018; 45:Suppl 20. S149–S161.
Article5. Ministry of Health, Labour and Welfare (JP). Survey of dental diseases [Internet]. Tokyo: Ministry of Health, Labour and Welfare;cited 2017 Jun 2. Available from: https://www.mhlw.go.jp/toukei/list/dl/62-28-01.pdf.6. Pink C, Kocher T, Meisel P, Dörr M, Markus MR, Jablonowski L, et al. Longitudinal effects of systemic inflammation markers on periodontitis. J Clin Periodontol. 2015; 42:988–997.
Article7. Shimada Y, Komatsu Y, Ikezawa-Suzuki I, Tai H, Sugita N, Yoshie H. The effect of periodontal treatment on serum leptin, interleukin-6, and C-reactive protein. J Periodontol. 2010; 81:1118–1123.
Article8. Budzyński J, Wiśniewska J, Ciecierski M, Kędzia A. Association between bacterial infection and peripheral vascular disease: a review. Int J Angiol. 2016; 25:3–13.
Article9. Aoyama N, Suzuki JI, Kobayashi N, Hanatani T, Ashigaki N, Yoshida A, et al. Periodontitis deteriorates peripheral arterial disease in Japanese population via enhanced systemic inflammation. Heart Vessels. 2017; 32:1314–1319.
Article10. Chen YW, Umeda M, Nagasawa T, Takeuchi Y, Huang Y, Inoue Y, et al. Periodontitis may increase the risk of peripheral arterial disease. Eur J Vasc Endovasc Surg. 2008; 35:153–158.
Article11. Aoyama N, Suzuki JI, Kobayashi N, Hanatani T, Ashigaki N, Yoshida A, et al. Detrimental effects of specific periodontopathic bacterial infection on tachyarrhythmia compared to bradyarrhythmia. BMC Cardiovasc Disord. 2017; 17:267.
Article12. Suzuki J, Aoyama N, Aoki M, Tada Y, Wakayama K, Akazawa H, et al. Incidence of periodontitis in Japanese patients with cardiovascular diseases: a comparison between abdominal aortic aneurysm and arrhythmia. Heart Vessels. 2015; 30:498–502.
Article13. Holm-Pedersen P, Avlund K, Morse DE, Stoltze K, Katz RV, Viitanen M, et al. Dental caries, periodontal disease, and cardiac arrhythmias in community-dwelling older persons aged 80 and older: is there a link? J Am Geriatr Soc. 2005; 53:430–437.
Article14. Maeda H, Fujimoto C, Haruki Y, Maeda T, Kokeguchi S, Petelin M, et al. Quantitative real-time PCR using TaqMan and SYBR green for Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis, Prevotella intermedia, tetQ gene and total bacteria. FEMS Immunol Med Microbiol. 2003; 39:81–86.
Article15. Ueno M, Izumi Y, Kawaguchi Y, Ikeda A, Iso H, Inoue M, et al. Prediagnostic plasma antibody levels to periodontopathic bacteria and risk of coronary heart disease. Int Heart J. 2012; 53:209–214.
Article16. Soejima H, Oe Y, Nakayama H, Matsuo K, Fukunaga T, Sugamura K, et al. Periodontal status and Prevotella intermedia antibody in acute coronary syndrome. Int J Cardiol. 2009; 137:304–306.
Article17. Aoyama N, Suzuki JI, Kumagai H, Ikeda Y, Akazawa H, Komuro I, et al. Specific periodontopathic bacterial infection affects hypertension in male cardiovascular disease patients. Heart Vessels. 2018; 33:198–204.
Article18. Aida J, Ando Y, Akhter R, Aoyama H, Masui M, Morita M. Reasons for permanent tooth extractions in Japan. J Epidemiol. 2006; 16:214–219.
Article19. Nesse W, Abbas F, van der Ploeg I, Spijkervet FK, Dijkstra PU, Vissink A. Periodontal inflamed surface area: quantifying inflammatory burden. J Clin Periodontol. 2008; 35:668–673.
Article20. Susanto H, Nesse W, Dijkstra PU, Hoedemaker E, van Reenen YH, Agustina D, et al. Periodontal inflamed surface area and C-reactive protein as predictors of HbA1c: a study in Indonesia. Clin Oral Investig. 2012; 16:1237–1242.
Article21. Hansson GK. Immune mechanisms in atherosclerosis. Arterioscler Thromb Vasc Biol. 2001; 21:1876–1890.
Article22. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005; 352:1685–1695.
Article23. Di Benedetto A, Gigante I, Colucci S, Grano M. Periodontal disease: linking the primary inflammation to bone loss. Clin Dev Immunol. 2013; 2013:503754.
Article24. Loos BG. Systemic markers of inflammation in periodontitis. J Periodontol. 2005; 76:2106–2115.
Article25. Loos BG, Craandijk J, Hoek FJ, Wertheim-van Dillen PM, van der Velden U. Elevation of systemic markers related to cardiovascular diseases in the peripheral blood of periodontitis patients. J Periodontol. 2000; 71:1528–1534.
Article26. Teeuw WJ, Slot DE, Susanto H, Gerdes VE, Abbas F, D'Aiuto F, et al. Treatment of periodontitis improves the atherosclerotic profile: a systematic review and meta-analysis. J Clin Periodontol. 2014; 41:70–79.
Article27. Aarabi G, Eberhard J, Reissmann DR, Heydecke G, Seedorf U. Interaction between periodontal disease and atherosclerotic vascular disease--fact or fiction? Atherosclerosis. 2015; 241:555–560.
Article28. Jepsen S, Caton JG, Albandar JM, Bissada NF, Bouchard P, Cortellini P, et al. Periodontal manifestations of systemic diseases and developmental and acquired conditions: consensus report of workgroup 3 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol. 2018; 89:Suppl 1. S237–S248.
Article29. Reynolds MA. Modifiable risk factors in periodontitis: at the intersection of aging and disease. Periodontol 2000. 2014; 64:7–19.
Article30. Holmstrup P, Damgaard C, Olsen I, Klinge B, Flyvbjerg A, Nielsen CH, et al. Comorbidity of periodontal disease: two sides of the same coin? An introduction for the clinician. J Oral Microbiol. 2017; 9:1332710.
Article31. Tonetti MS, D'Aiuto F, Nibali L, Donald A, Storry C, Parkar M, et al. Treatment of periodontitis and endothelial function. N Engl J Med. 2007; 356:911–920.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The comparison of IL-6, elastase and alpha1-PI expressions in human chronic periodontitis with type 2 diabetes mellitus
- Galectin-3 as a possible link between periodontitis and chronic kidney disease: a cross-sectional study
- Epigenetic biomarkers: a step forward for understanding periodontitis
- Microbiological links between periodontitis and systemic diseases: a brief review
- Diabetes and Periodontal Disease