Ann Clin Microbiol.
2018 Dec;21(4):80-85. 10.5145/ACM.2018.21.4.80.
Inhibitory Effect of Metal Surface on the Antimicrobial Resistance Microorganism
- Affiliations
-
- 1Department of Food Science and Technology, Sunchon National University, Suncheon, Korea.
- 2Department of Laboratory Medicine, The Catholic University of Korea, Uijeongbu St. Mary's Hospital, Seoul, Korea. hkl@catholic.ac.kr
- 3Department of Laboratory Medicine, The Catholic University of Korea, Seoul St. Mary's Hospital, Seoul, Korea.
- KMID: 2429651
- DOI: http://doi.org/10.5145/ACM.2018.21.4.80
Abstract
- BACKGROUND
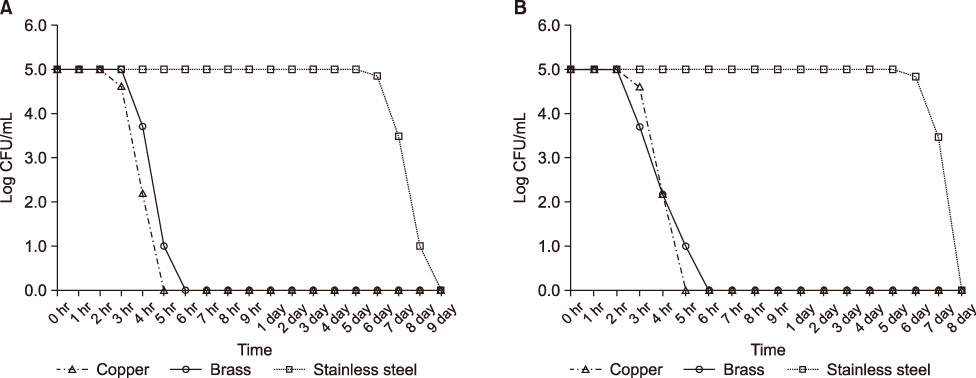
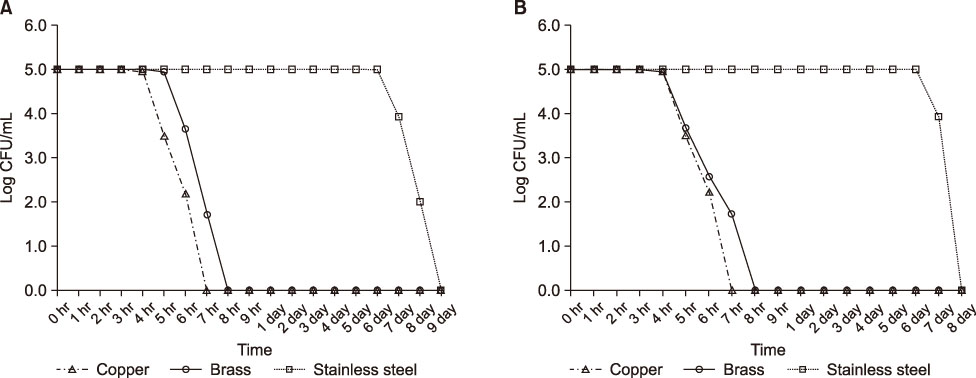
The aim of this study was to comparatively evaluate the bactericidal effects of copper, brass (copper 78%, tin 22%), and stainless steel against methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus faecium (VREFM), and multidrug-resistant Pseudomonas aeruginosa (MRPA).
METHODS
The isolates (MRSA, VREFM, MRPA) used in this study were mixed wild type 3 strains isolated from patients treated at Uijeongbu St. Mary's Hospital in 2017. These strains showed patterns of multidrug resistance. The lyophilized strains were inoculated into and incubated for 24 hr in tryptic soy broth at 35℃. The initial bacterial inoculum concentration was adjusted to 105 CFU/mL. A 100-mL bacterial suspension was incubated in containers made of brass (copper 78%, tin 22%), copper (above 99% purity), and stainless steel at 35℃. Viable counts of bacteria strains were measured for 9 days.
RESULTS
In this study, the bactericidal effects of copper and brass on MRSA, VREFM, and MRPA were verified. The bactericidal effect of stainless steel was much weaker than those of copper and brass. The bactericidal effect was stronger on MRPA than on MRSA or VREFM.
CONCLUSION
To prevent cross infection of multidrug resistant bacteria in hospitals, further studies of longer duration are needed for testing of copper materials on objects such as door knobs, faucets, and bed rails.
MeSH Terms
Figure
Reference
-
1. Kim JM. Antibiotic resistance of Helicobacter pylori isolated from Korean patients. Korean J Gastroenterol. 2006; 47:337–349.2. Jevons MP. “Celbenin” - resistant staphylococci. Br Med J. 1961; 1:124–125.
Article3. Grass G, Rensing C, Solioz M. Metallic copper as an antimicrobial surface. Appl Environ Microbiol. 2011; 77:1541–1547.
Article4. Burton DC, Edwards JR, Horan TC, Jernigan JA, Fridkin SK. Methicillin-resistant Staphylococcus aureus central line-associated bloodstream infections in US intensive care units, 1997–2007. JAMA. 2009; 301:727–736.5. Lee H, Yong D, Lee K, Hong SG, Kim EC, Jeong SH, et al. Antimicrobial resistance of clinically important bacteria isolated from 12 hospitals in Korea in 2004. Korean J Clin Microbiol. 2005; 8:66–73.6. Moon HW, Kim HJ, Hur M, Yun YM. Antimicrobial susceptibility profiles of Staphylococcus aureus isolates classified according to their origin in a tertiary hospital in Korea. Am J Infect Control. 2014; 42:1340–1342.7. Stosor V, Noskin GA, Peterson LR. The management and prevention of vancomycin-resistant enterococci. Infect Med. 1996; 13:487–488. 4938. Breathnach AS, Cubbon MD, Karunaharan RN, Pope CF, Planche TD. Multidrug-resistant Pseudomonas aeruginosa outbreaks in two hospitals: association with contaminated hospital waste-water systems. J Hosp Infect. 2012; 82:19–24.9. Weber DJ, Rutala WA. Understanding and preventing transmission of healthcare-associated pathogens due to the contaminated hospital environment. Infect Control Hosp Epidemiol. 2013; 34:449–452.
Article10. Kim YA, Lee H, Lee K. Contamination of the hospital environmental by pathogenic bacteria and infection control. Korean J Nosocomial Infect Control. 2015; 20:1–6.
Article11. Bae JY, Kang CK, Choi SJ, Lee E, Choe PG, Park WB, et al. Sudden deaths of neonates receiving intravenous infusion of lipid emulsion contaminated with Citrobacter freundii. J Korean Med Sci. 2018; 33:e97.
Article12. Dollwet HHA, Sorenson JRJ. Historic uses of copper compounds in medicine. J Trace Elem Med. 1985; 2:80–87.13. Kuhn PJ. Door knobs: a source of nosocomial infection? Diagnostic Medicine;last visited 12 November 2018. https://www.antimicrobialcopper.org/sites/default/files/upload/media-library/files/pdfs/uk/scientific_literature/kuhn-doorknob.pdf [Online].14. Kusumaningrum HD, Riboldi G, Hazeleger WC, Beumer RR. Survival of foodborne pathogens on stainless steel surfaces and cross-contamination to foods. Int J Food Microbiol. 2003; 85:227–236.
Article15. Agarwala M, Choudhury B, Yadav RN. Comparative study of antibiofilm activity of copper oxide and iron oxide nanoparticles against multidrug resistant biofilm forming uropathogens. Indian J Microbiol. 2014; 54:365–368.
Article16. Gould SWJ, Fielder MD, Kelly AF, Morgan M, Kenny J, Naughton DP. The antimicrobial properties of copper surfaces against a range of important nosocomial pathogens. Ann Microbiol. 2009; 59:151–156.
Article17. Carson KC, Bartlett JG, Tan TJ, Riley TV. In vitro susceptibility of methicillin-resistant Staphylococcus aureus and methicillin-susceptible Staphylococcus aureus to a new antimicrobial, copper silicate. Antimicrob Agents Chemother. 2007; 51:4505–4507.18. Lee EJ, Park JH. Inactivation activity of bronze alloy Yugi for reduction of cross-contamination of food-borne pathogen in food processing. J Food Hyg Saf. 2008; 23:309–313.19. Mikolay A, Huggett S, Tikana L, Grass G, Braun J, Nies DH. Survival of bacteria on metallic copper surfaces in a hospital trial. Appl Microbiol Biotechnol. 2010; 87:1875–1879.
Article20. Casey AL, Adams D, Karpanen TJ, Lambert PA, Cookson BD, Nightingale P, et al. Role of copper in reducing hospital environment contamination. J Hosp Infect. 2010; 74:72–77.
Article21. Jung MK, Lee MY, Park JH. Inhibitory effect of cupric ion diffused from brass ware on the growth of E. coli O157:H7, S. typhimurium, S. aureus, and B. cereus. Food Sci Biotechnol. 2004; 13:680–683.22. CLSI. Performance standards for antimicrobial susceptibility testing: twenty-third informational supplement. CLSI document M100-S23. Wayne, PA: Clinical and Laboratory Standards Institutes;2013.23. Weinstein RA. Epidemiology and control of nosocomial infections in adult intensive care units. Am J Med. 1991; 91:179S–184S.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A scanning electron microscopic study on the corrosion resistance of chemically and thermally recycled metal brackets
- Acute uncomplicated cystitis in the emergency department: prevalence of antimicrobial resistance among uropathogens and appropriate antimicrobial treatment
- Antimicrobial Resistance for Chlamydia Trachomatis Genital Infection during Pregnancy in Japan
- Isolation of Causative Microorganism and Antimicrobial Susceptibility Test in Impetigo Developed in the Past Four Years
- Antimicrobial effect of calcium hydroxide as an intracanal medicament in root canal treatment: a literature review - Part II. in vivo studies