Int J Stem Cells.
2018 Nov;11(2):216-226. 10.15283/ijsc18019.
Comparative Study on Acellular Dermal Graft Versus Propylene Mesh Both Either Loaded or Unloaded with BM-MSCs in Healing of Skull Bone Defect in Rats: Histological and Immunohistochemical Study
- Affiliations
-
- 1Department of Histology and Cell Biology, Faculty of Medicine Ain Shams University, Cairo, Egypt. ghada.hamam@yahoo.com
- 2Medical Research Center, Ain Shams University, Cairo, Egypt.
- KMID: 2429554
- DOI: http://doi.org/10.15283/ijsc18019
Abstract
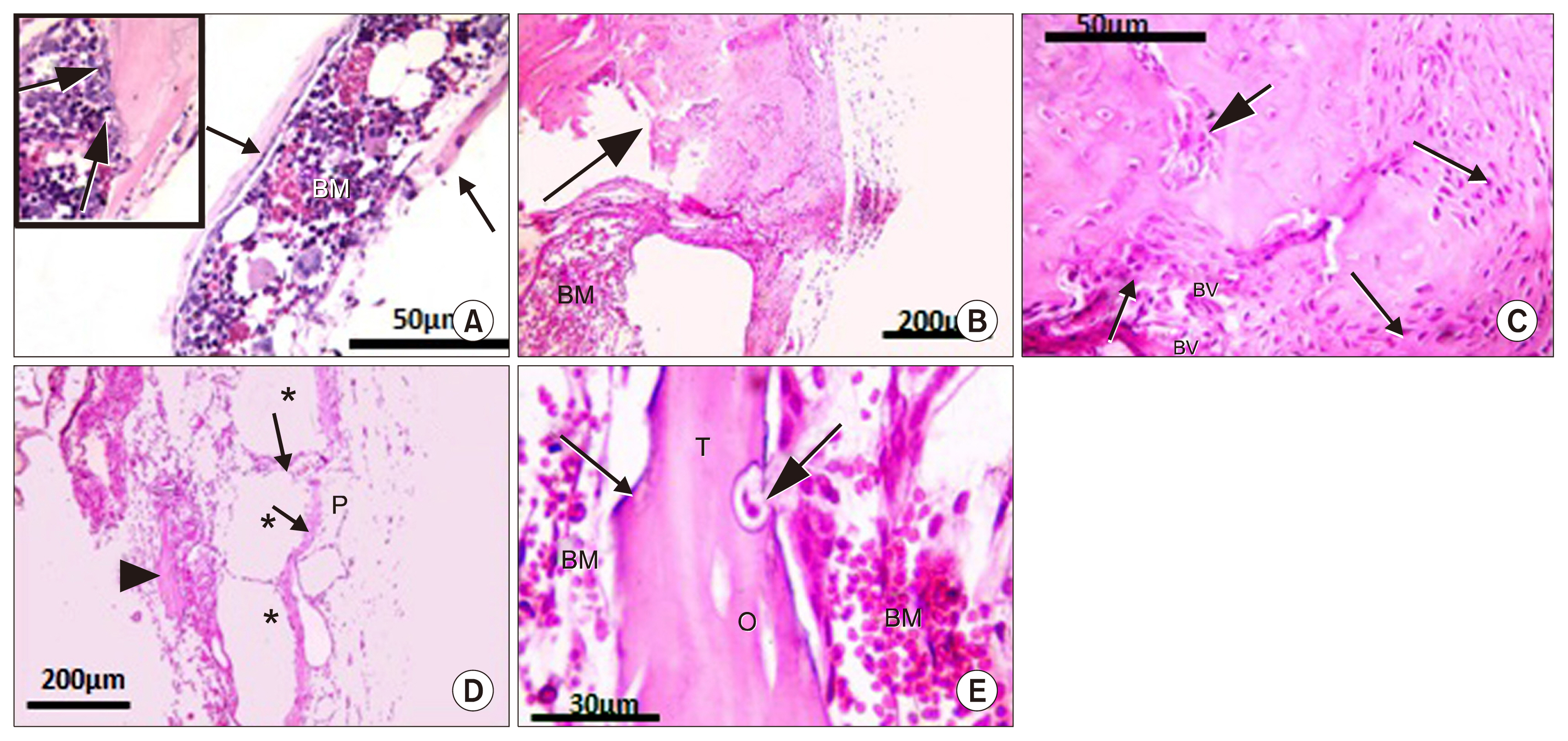
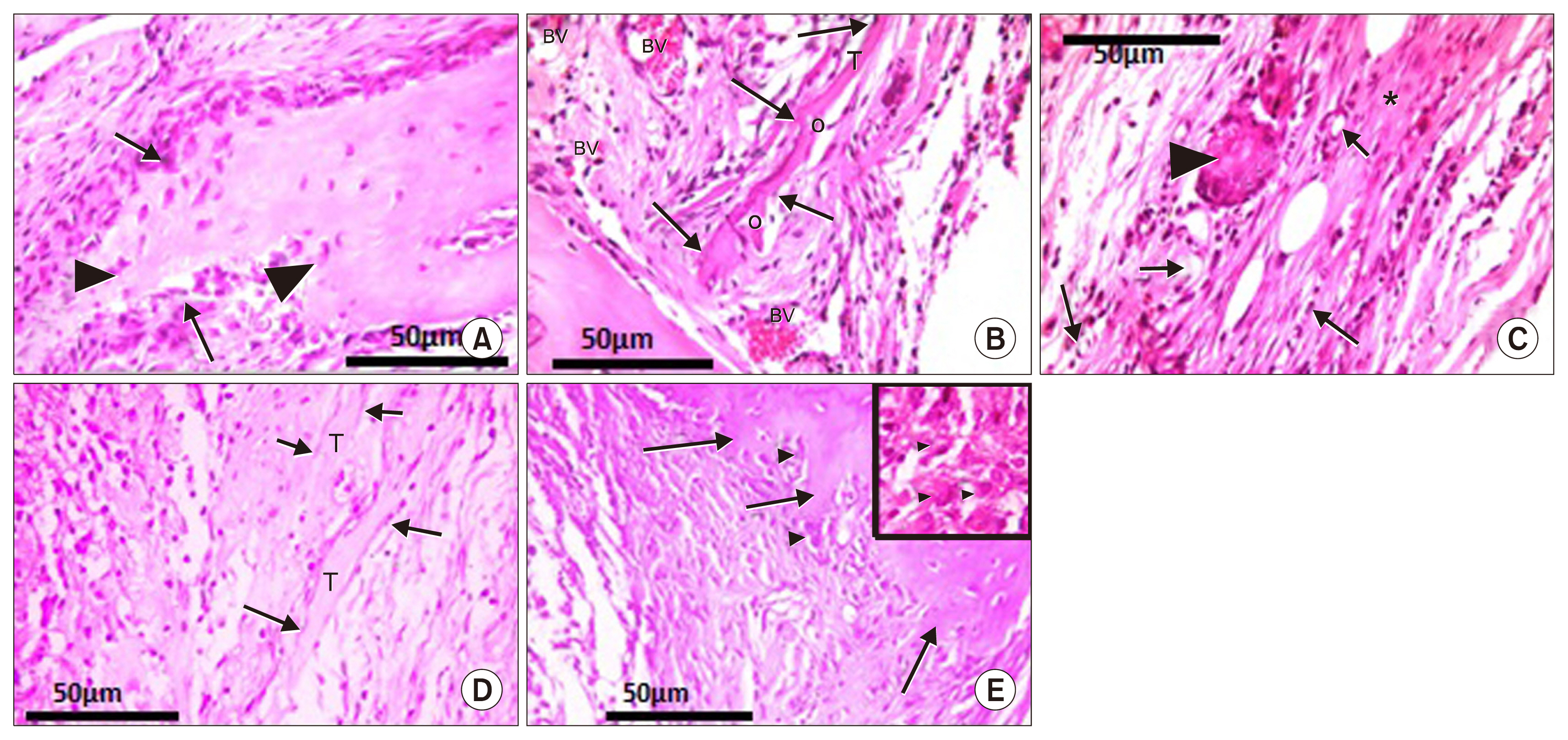
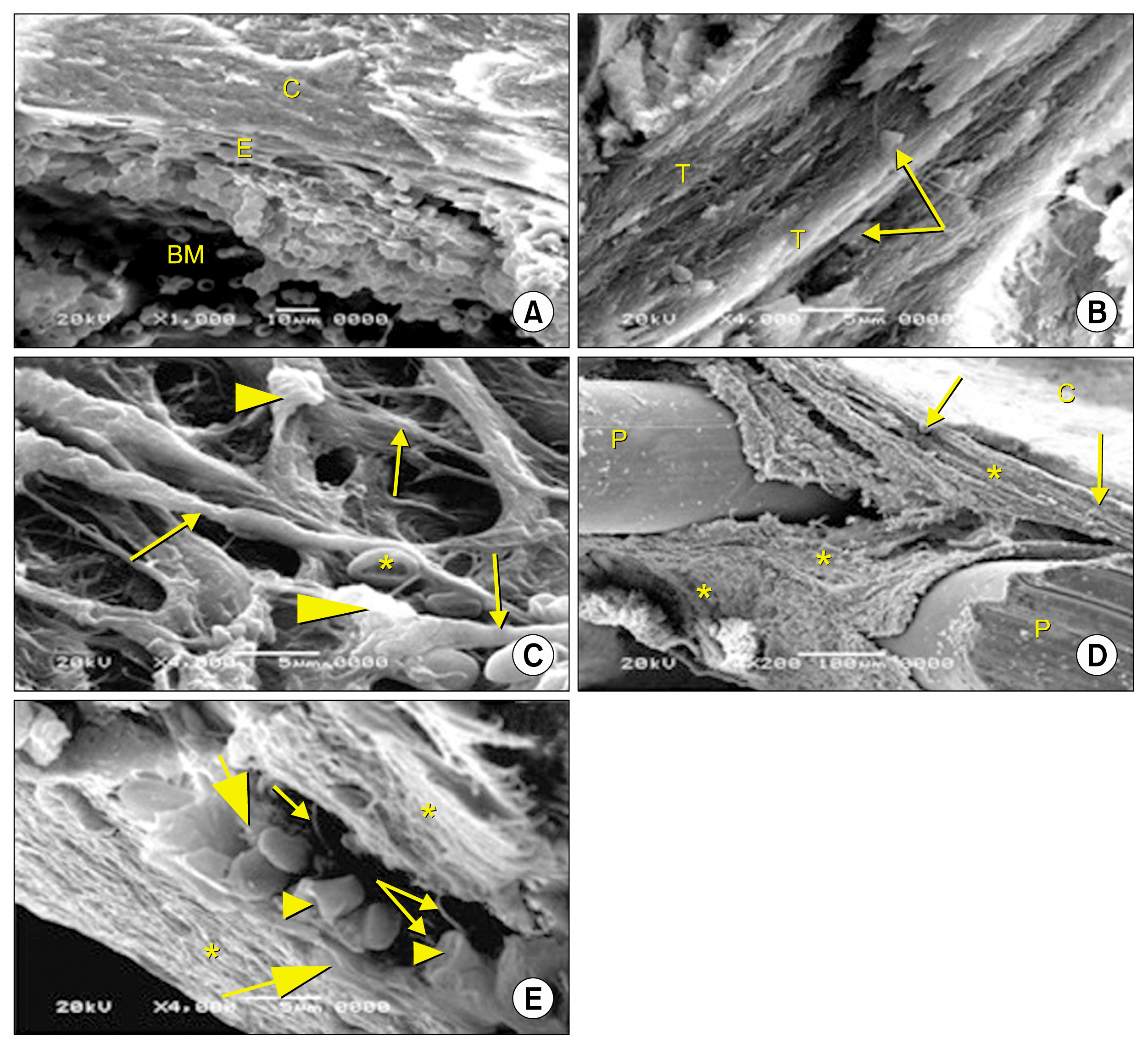
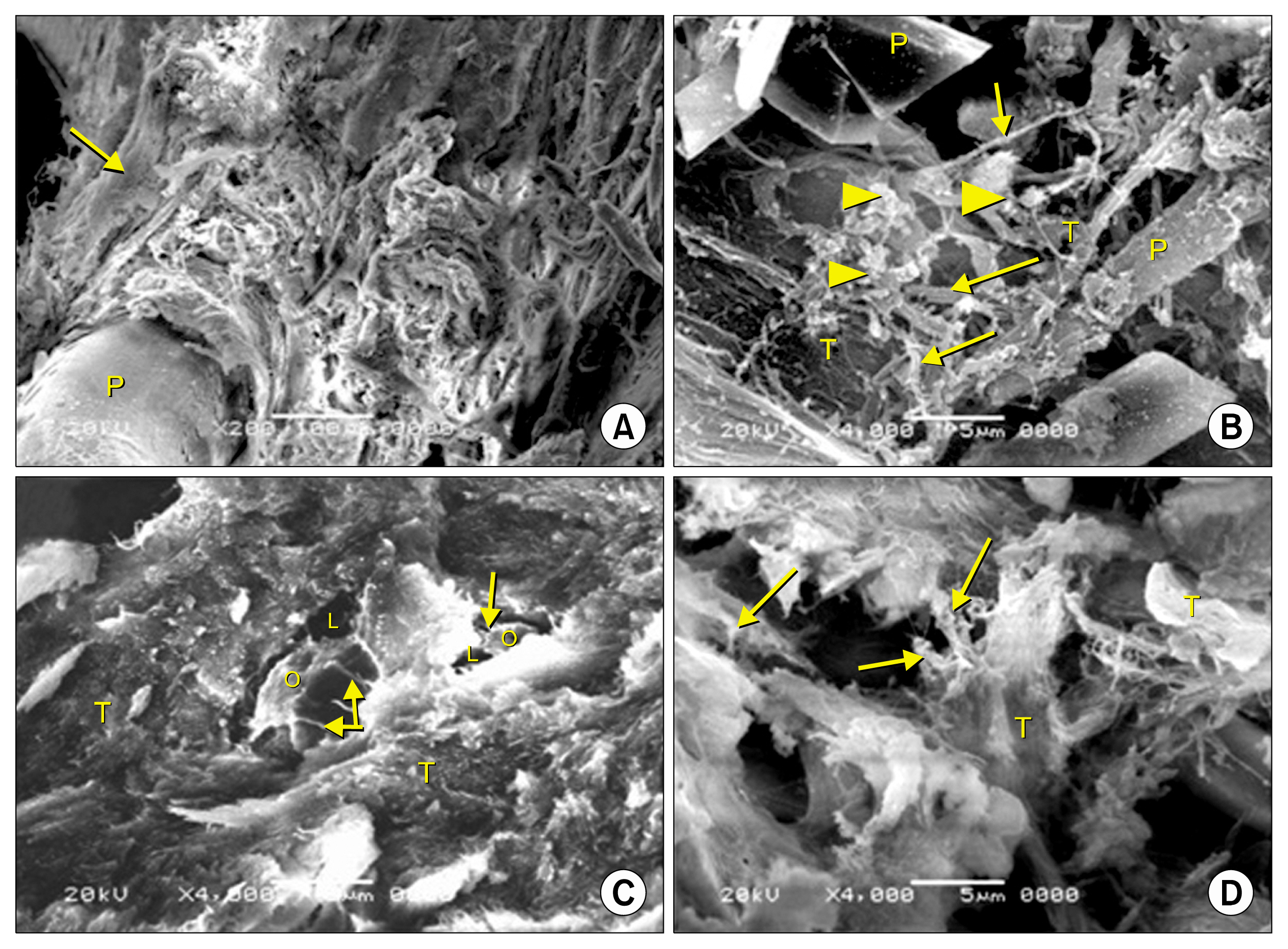
- Bone defect occurs as a consequence of many conditions. Diseased bones don't heal properly and defects in face area need proper bone reconstruction to avoid psychological and social problems. Tissue engineering is an emerging new modality of treatment. We thought to study different methods to fill skull bone defect in rats in order to find the most safe and effective method. So, this study was designed to evaluate the efficacy of acellular dermal graft (ADM) versus propylene mesh both either loaded or unloaded with bone marrow derived mesenchymal stem cells (BM-MSCs) in healing of skull bone defect of a 5 mm diameter. The study included 36 adult male Wistar albino rats that were divided into three groups according to the way of filling skull bone defect. Group I: Ia (sham control), Ib (negative control). Group II: IIa (unseeded propylene), IIb (seeded propylene) and Group III: IIIa (unseeded ADM), IIIb (seeded ADM). The trephine operation was done on the left parietal bone. Specimens were collected four weeks postoperative and processed for H&E, osteopontin immunohistochemistry and scanning electron microscope. Morphometric and statistical analysis were also performed. After studying the results of the experiment, we found that propylene mesh and ADM were suitable scaffolds that could support new bone formation in clavarial bone defect. Healing of skull bone defect was better in rats that received seeded scaffolds more than rats with unseeded scaffolds. The seeded ADM showed significant increase in bone forming activity as confirmed by histomorphometric and statistical results.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Lewandrowski KU, Gresser JD, Wise DL, Trantol DJ. Bioresorbable bone graft substitutes of different osteoconductivities: a histologic evaluation of osteointegration of poly(propylene glycol-co-fumaric acid)-based cement implants in rats. Biomaterials. 2000; 21:757–764. DOI: 10.1016/S0142-9612(99)00179-9. PMID: 10721744.
Article2. Javaid MA, Kaartinen MT. Mesenchymal stem cell-based bone tissue engineering. International Dental Journal of Students Research. 2013; 1:24–35.3. Giannoudis PV, Dinopoulos H, Tsiridis E. Bone substitutes: an update. Injury. 2005; 36(Suppl 3):S20–S27. DOI: 10.1016/j.injury.2005.07.029. PMID: 16188545.
Article4. Chatterjea A, Meijer G, van Blitterswijk C, de Boer J. Clinical application of human mesenchymal stromal cells for bone tissue engineering. Stem Cells Int. 2010; DOI: 10.4061/2010/215625. PMID: 21113294. PMCID: 2989379.
Article5. Arnold S, Valerie J. Implant materials. Thorne CH, editor. Grabb and Smith’s Plastic Surgery. 6th ed. Lippincott Williams & Wilkins, a Wolters Kluwer business;2007. p. 58–65.6. Kaigler D, Pagni G, Park CH, Braun TM, Holman LA, Yi E, Tarle SA, Bartel RL, Giannobile WV. Stem cell therapy for craniofacial bone regeneration: a randomized, controlled feasibility trial. Cell Transplant. 2013; 22:767–777. DOI: 10.3727/096368912X652968.
Article7. Psarras S, Mavroidis M, Sanoudou D, Davos CH, Xanthou G, Varela AE, Panoutsakopoulou V, Capetanaki Y. Regulation of adverse remodelling by osteopontin in a genetic heart failure model. Eur Heart J. 2012; 33:1954–1963. DOI: 10.1093/eurheartj/ehr119.
Article8. Ferreira GR, Cestari TM, Granjeiro JM, Taga R. Lack of repair of rat skull critical size defect treated with bovine morphometric protein bound to microgranular bioabsorbable hydroxyapatite. Braz Dent J. 2004; 15:175–180. DOI: 10.1590/S0103-64402004000300002.
Article9. Spicer PP, Kretlow JD, Young S, Jansen JA, Kasper FK, Mikos AG. Evaluation of bone regeneration using the rat critical size calvarial defect. Nat Protoc. 2012; 7:1918–1929. DOI: 10.1038/nprot.2012.113. PMID: 23018195. PMCID: 3513397.
Article10. Mokal NJ, Desai MF. Calvarial reconstruction using high-density porous polyethylene cranial hemispheres. Indian J Plast Surg. 2011; 44:422–431. DOI: 10.4103/0970-0358.90812.
Article11. Walter JB, Talbot IC. Walter and Israel general pathology. 7th ed. British Library;1996. p. 184–415.12. Xu Y, Zhang G, Chang Y, Qiu YX, Wang C. The preparation of acellular dermal matrices by freeze-thawing and ultrasonication process and the evaluation of its antigenicity. Cell Biochem Biophys. 2015; 73:27–33. DOI: 10.1007/s12013-015-0569-9. PMID: 25649614.
Article13. McFarlin K, Gao X, Liu YB, Dulchavsky DS, Kwon D, Arbab AS, Bansal M, Li Y, Chopp M, Dulchavsky SA, Gautam SC. Bone marrow-derived mesenchymal stromal cells accelerate wound healing in the rat. Wound Repair Regen. 2006; 14:471–478. DOI: 10.1111/j.1743-6109.2006.00153.x. PMID: 16939576.
Article14. Li H, Fu X, Ouyang Y, Cai C, Wang J, Sun T. Adult bone-marrow-derived mesenchymal stem cells contribute to wound healing of skin appendages. Cell Tissue Res. 2006; 326:725–736. DOI: 10.1007/s00441-006-0270-9. PMID: 16906419.
Article15. Wang Q, Jin Y, Deng X, Liu H, Pang H, Shi P, Zhan Z. Second-harmonic generation microscopy for assessment of mesenchymal stem cell-seeded acellular dermal matrix in wound-healing. Biomaterials. 2015; 53:659–668. DOI: 10.1016/j.biomaterials.2015.03.011. PMID: 25890761.
Article16. Suvarna K, Layton C, Bancroft J. Theory and practice of histological techniques. 7th ed. USA: Churchill Livingston;2013.17. Armitage P, Berry G. Statistical methods in medical research. 3rd ed. Oxford: Blackwell Scientific Publications;1994. p. 12–48.18. Dorozhkin SV. Calcium orthophosphate-containing biocomposites and hybrid biomaterials for biomedical applications. J Funct Biomater. 2015; 6:708–832. DOI: 10.3390/jfb6030708. PMID: 26262645. PMCID: 4598679.
Article19. Ferreira LB, Bradaschia-Correa V, Moreira MM, Marques ND, Arana-Chavez VE. Evaluation of bone repair of critical size defects treated with simvastatin-loaded poly(lactic-coglycolic acid) microspheres in rat calvaria. J Biomater Appl. 2015; 29:965–976. DOI: 10.1177/0885328214550897.
Article20. Sohn JY, Park JC, Um YJ, Jung UW, Kim CS, Cho KS, Choi SH. Spontaneous healing capacity of rabbit cranial defects of various sizes. J Periodontal Implant Sci. 2010; 40:180–187. DOI: 10.5051/jpis.2010.40.4.180. PMID: 20827327. PMCID: 2931306.
Article21. Claes L, Recknagel S, Ignatius A. Fracture healing under healthy and inflammatory conditions. Nat Rev Rheumatol. 2012; 8:133–143. DOI: 10.1038/nrrheum.2012.1. PMID: 22293759.
Article22. Galindo P, Hernndez P, Padial M, Luisa M, Crespo V, O’Valle F. Osteopontin expression in an organic bovine bone Immunohistochemical osteopontin expression in bone xenograft in clinical series of maxillary sinus lift. J of Oral Sci & Rehab. 2015; 1:42–50.23. Cunniffe GM, Díaz-Payno PJ, Ramey JS, Mahon OR, Dunne A, Thompson EM, O’Brien FJ, Kelly DJ. Growth plate extracellular matrix-derived scaffolds for large bone defect healing. Eur Cell Mater. 2017; 33:130–142. DOI: 10.22203/eCM.v033a10. PMID: 28197989.
Article24. Zogbi L. The use of biomaterials to treat abdominal hernias. Biomaterials Applications for Nanomedicine. Pignatello Rosario, editor. 978-953-307-661-4. InTech;2011. p. 359–382.
Article25. Wolf K, Alexander S, Schacht V, Coussens LM, von Andrian UH, van Rheenen J, Deryugina E, Friedl P. Collagen-based cell migration models in vitro and in vivo. Semin Cell Dev Biol. 2009; 20:931–941. DOI: 10.1016/j.semcdb.2009.08.005. PMID: 19682592. PMCID: 4021709.
Article26. Petite H, Viateau V, Bensaïd W, Meunier A, de Pollak C, Bourguignon M, Oudina K, Sedel L, Guillemin G. Tissue-engineered bone regeneration. Nat Biotechnol. 2000; 18:959–963. DOI: 10.1038/79449. PMID: 10973216.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Healing patterns of the acellular dermal matrix depend on graft method in the rabbit ears
- Usefulness of acellular dermal matrix graft on the tissue regeneration in rabbits
- Effects of Recombinant Human Bone Morphogenetic Protein-2 loaded Acellular Dermal Matrix on Bone Formation
- Autogenous Living Bone Graft and Dead Bone Graft in the Rabbit Tibia
- Role of Bone Marrow Mesenchymal Stem Cells in the Treatment of CCL4 Induced Liver Fibrosis in Albino Rats: A Histological and Immunohistochemical Study