Transl Clin Pharmacol.
2018 Dec;26(4):150-154. 10.12793/tcp.2018.26.4.150.
Pharmacodynamic principles and target concentration intervention
- Affiliations
-
- 1Department of Pharmacology & Clinical Pharmacology, University of Auckland, Auckland, New Zealand. n.holford@auckland.ac.nz
- KMID: 2429509
- DOI: http://doi.org/10.12793/tcp.2018.26.4.150
Abstract
- This tutorial reviews the principles of dose individualisation with an emphasis on target concentration intervention (TCI). Once a target effect is chosen then pharmacodynamics can predict the target concentration and pharmacokinetics can predict the target dose to achieve the required response. Dose individualisation can be considered at three levels: population, group and individual. Population dosing, also known as fixed dosing or "one size fits all" is often used but is poor clinical pharmacology; group dosing uses patient features such as weight, organ function and co-medication to adjust the dose for a typical patient; individual dosing uses observations of patient response to inform about pharmacokinetic and pharmacodynamics in the individual and use these individual differences to individualise dose.
Keyword
Figure
Reference
-
1. Holford NH. Target concentration intervention: beyond Y2K. Br J Clin Pharmacol. 1999; 48:9–13.
Article2. Holford N. Pharmacodynamic principles and the time course of immediate drug effects. Transl Clin Pharmacol. 2017; 25:157–161.
Article3. Holford N, Yim DS. Clearance. Transl Clin Pharmacol. 2015; 23:42–45.
Article4. Holford N, Yim DS. Volume of distribution. Transl Clin Pharmacol. 2016; 24:74–77.
Article5. New Zealand Formulary. Morphine monograph. 2019. https://nzf.org.nz/nzf_2515.6. Holford NH, Ma SC, Anderson BJ. Prediction of morphine dose in humans. Paediatr Anaesth. 2012; 22:209–222. DOI: 10.1111/j.1460-9592.2011.03782.x.
Article7. Holford N, Black P, Couch R, Kennedy J, Briant R. Theophylline target concentration in severe airways obstruction - 10 or 20 mg/L? A randomised concentration-controlled trial. Clin Pharmacokinet. 1993; 25:495–505.
Article8. Holford N, Hashimoto Y, Sheiner LB. Time and theophylline concentration help explain the recovery of peak flow following acute airways obstruction. Population analysis of a randomised concentration controlled trial. Clin Pharmacokinet. 1993; 25:506–515.
Article9. Holford N. Pharmacokinetic variability due to environmental differences. Transl Clin Pharmacol. 2017; 25:59–62.
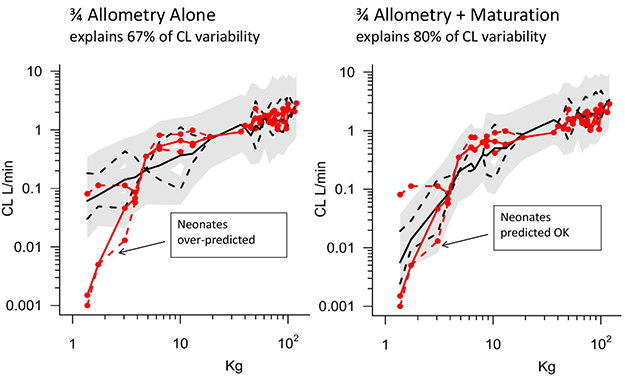
Article10. Peeters MY, Allegaert K, Blussé van Oud-Alblas HJ, Cella M, Tibboel D, Danhof M, et al. Prediction of propofol clearance in children from an allometric model developed in rats, children and adults versus a 0.75 fixed-exponent allometric model. Clin Pharmacokinet. 2010; 49:269–275. DOI: 10.2165/11319350-000000000-00000.
Article11. Holford NH, Buclin T. Safe and effective variability - a criterion for dose individualization. Ther Drug Monit. 2012; 34:565–568. DOI: 10.1097/FTD.0b013e31826aabc3.12. Sheiner L, Tozer T. Clinical pharmacokinetics: The use of plasma concentrations of drugs. In : Melmon K, Morelli H, editors. Clinical Pharmacology: Basic Principles of Therapeutics. New York: Macmillan;1978. p. 71–109.13. Sheiner LB, Rosenberg B, Melmon KL. Modelling of individual pharmacokinetics for computer-aided drug dosage. Comput Biomed Res. 1972; 5:441–459.
Article14. Hale MD, Nicholls AJ, Bullingham RE, Hené R, Hoitsma A, Squifflet JP, et al. The pharmacokinetic-pharmacodynamic relationship for mycophenolate mofetil in renal transplantation. Clin Pharmacol Ther. 1998; 64:672–683.
Article15. Le Meur Y, Büchler M, Thierry A, Caillard S, Villemain F, Lavaud S, et al. Individualized mycophenolate mofetil dosing based on drug exposure significantly improves patient outcomes after renal transplantation. Am J Transplant. 2007; 7:2496–2503.
Article16. van Gelder T, Hilbrands LB, Vanrenterghem Y, Weimar W, de Fijter JW, Squifflet JP, et al. A randomized double-blind, multicenter plasma concentration controlled study of the safety and efficacy of oral mycophenolate mofetil for the prevention of acute rejection after kidney transplantation. Transplantation. 1999; 68:261–266.
Article17. Gaston RS, Kaplan B, Shah T, Cibrik D, Shaw LM, Angelis M, et al. Fixed- or controlled-dose mycophenolate mofetil with standard- or reduced-dose calcineurin inhibitors: the Opticept trial. Am J Transplant. 2009; 9:1607–1619. DOI: 10.1111/j.1600-6143.2009.02668.x.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Obesity and anesthetic pharmacology: simulation of target-controlled infusion models of propofol and remifentanil
- Basic Principles of Drug Interaction
- Pharmacodynamic principles and the time course of delayed and cumulative drug effects
- Pharmacodynamic principles and the time course of immediate drug effects
- A semi-compartmental model describing the pharmacokinetic-pharmacodynamic relationship