Anesth Pain Med.
2020 Jan;15(1):1-7. 10.17085/apm.2020.15.1.1.
A semi-compartmental model describing the pharmacokinetic-pharmacodynamic relationship
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Inje University Busan Paik Hospital, Inje University College of Medicine, Busan, Korea
- KMID: 2500448
- DOI: http://doi.org/10.17085/apm.2020.15.1.1
Abstract
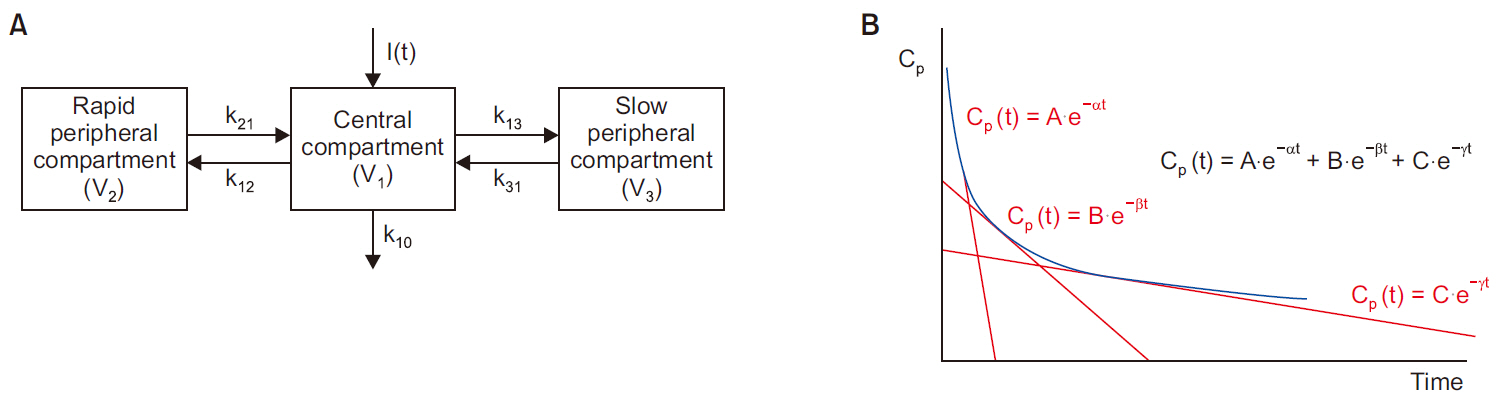
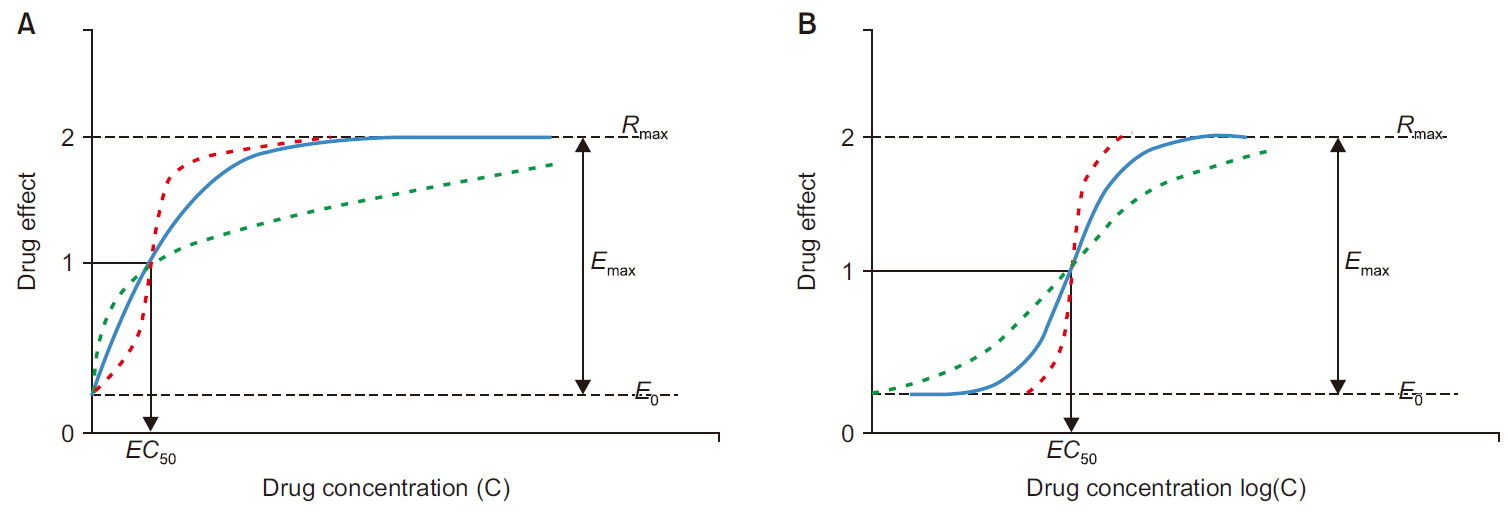
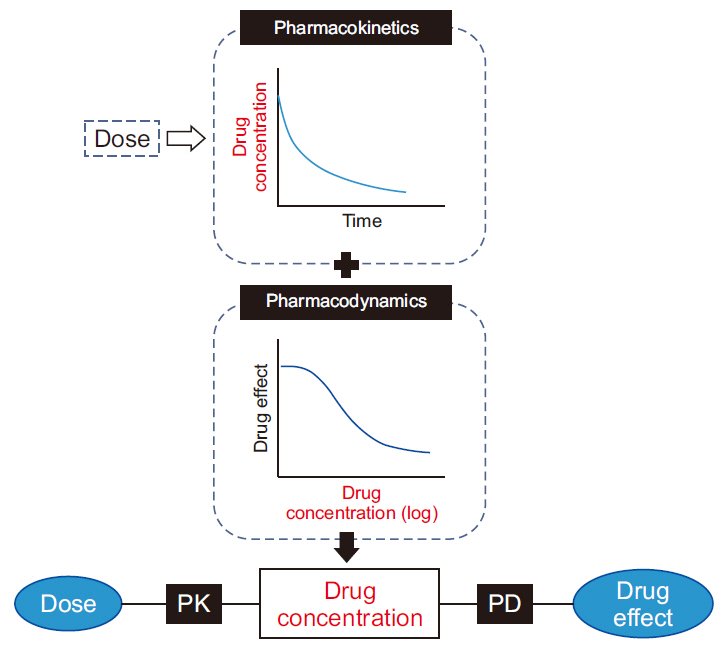
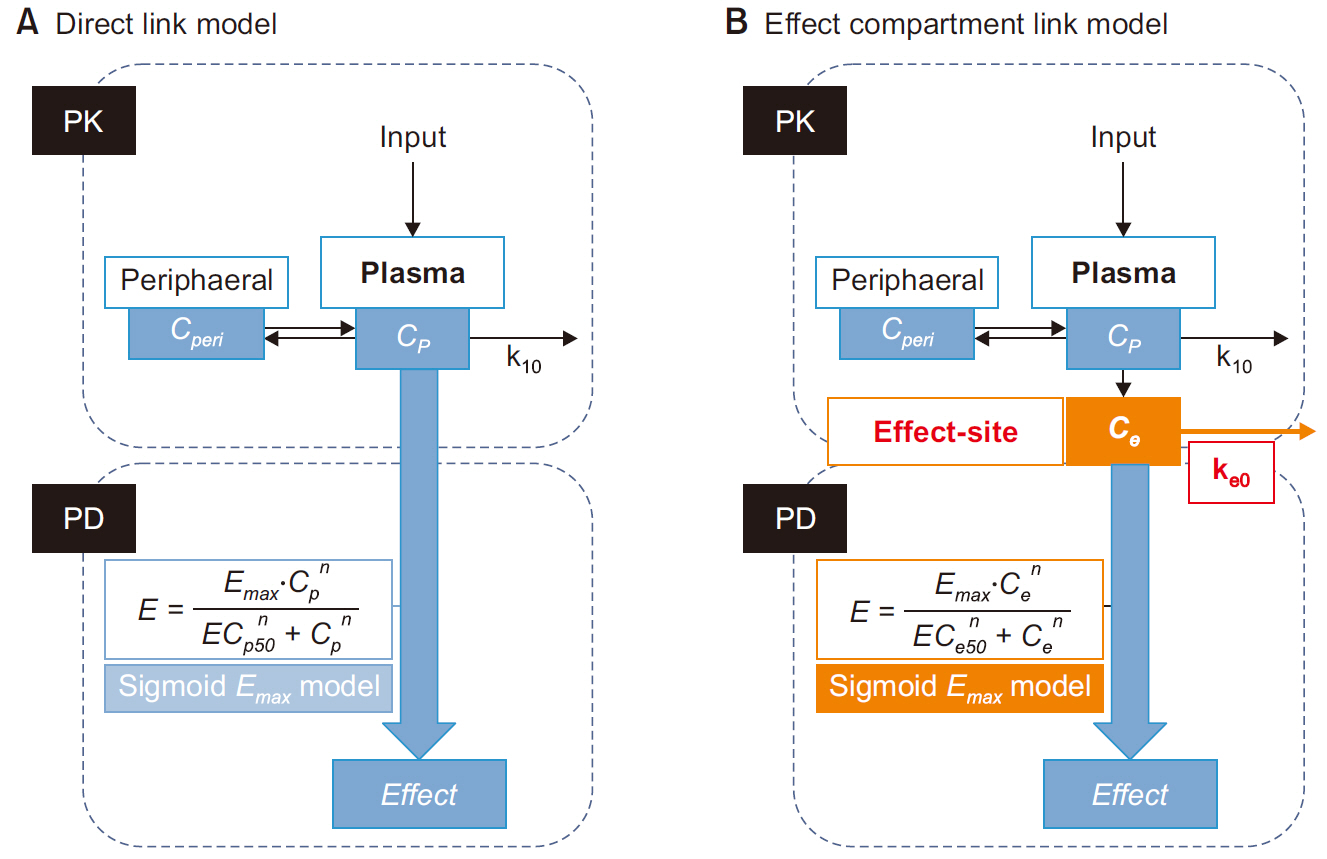
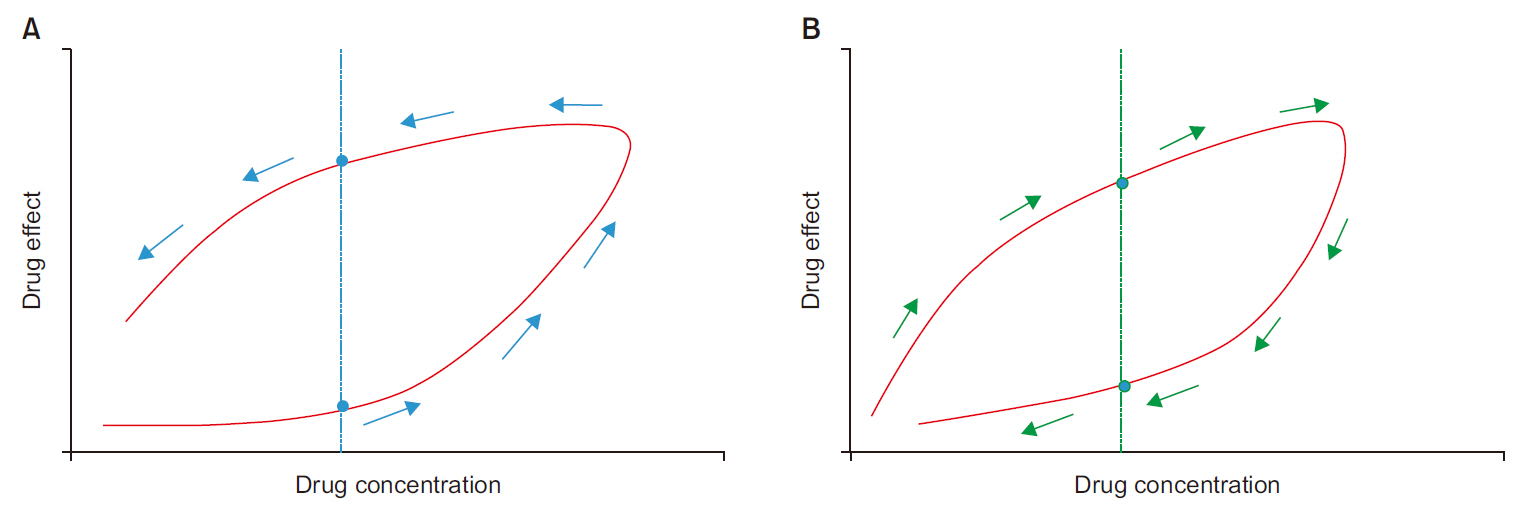
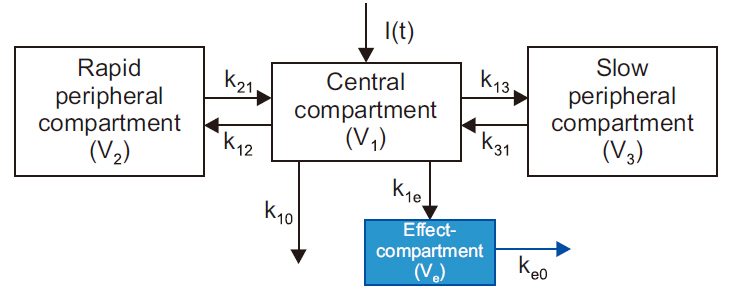
- Frequently, we encounter the phenomenon of hysteresis in kinetic-dynamic modeling. The hysteresis loop in the concentration-effect curve suggests a time discrepancy caused by various pharmacokinetic and pharmacodynamic factors. To collapse the hysteresis loop and to simplify the concentration-effect relationship, several kinetic-dynamic modeling approaches including the effect compartment link model, turnover model (indirect response model), and tolerance/rebound model, have been used. The semicompartmental model is one method to describe the hysteresis of the pharmacokineticpharmacodynamic relationship. Furthermore, this semi-compartmental model differs from other models (full parametric approaches) as it does not require pharmacokinetic parameters to estimate pharmacodynamic parameters and ke0. Therefore, we could employ a semi-compartmental approach in case it is difficult to apply the compartment model to pharmacokinetic data, as required for the pharmacodynamic analysis of inhalational anesthetics.
Figure
Reference
-
Riviere JE., Gabrielsson J., Fink M., Mochel J. 2016. Mathematical modeling and simulation in animal health. Part I: moving beyond pharmacokinetics. J Vet Pharmacol Ther. 39:213–23. DOI: 10.1111/jvp.12278. PMID: 26592724.Gabrielsson J., Weiner D. 2016. Pharmacokinetic and pharmacodynamic data analysis: concepts and applications. . 5th ed. Apotekarsocieteten;Stockholm:Derendorf H., Meibohm B. 1999. Modeling of pharmacokinetic/pharmacodynamic (PK/PD) relationships: concepts and perspectives. Pharm Res. 16:176–85. DOI: 10.1023/A:1011907920641. PMID: 10100300.Holford NH., Sheiner LB. 1981. Understanding the dose-effect relationship: clinical application of pharmacokinetic-pharmacodynamic models. Clin Pharmacokinet. 6:429–53. DOI: 10.2165/00003088-198106060-00002. PMID: 7032803.Meibohm B., Derendorf H. 1997. Basic concepts of pharmacokinetic/pharmacodynamic (PK/PD) modelling. Int J Clin Pharmacol Ther. 35:401–13. PMID: 9352388.Louizos C., Yáñez JA., Forrest ML., Davies NM. 2014. Understanding the hysteresis loop conundrum in pharmacokinetic/pharmacodynamic relationships. J Pharm Pharm Sci. 17:34–91. DOI: 10.18433/J3GP53. PMID: 24735761. PMCID: PMC4332569.Gabrielsson J., Weiner D. Gabrielsson J, Weiner D, editors. 2016. Pharmacodynamic concepts. Pharmacokinetic and Pharmacodynamic Data Analysis: Concepts and Applications. 5th ed. Apotekarsocieteten;Stockholm: p. 199–332.Gabrielsson J., Weiner D. Gabrielsson J, Weiner D, editors. 2016. Pharmacokinetic concepts: multi-compartment model. Pharmacokinetic and Pharmacodynamic Data Analysis: Concepts and Applications. 5th ed. Apotekarsocieteten;Stockholm: p. 57–72.Han DW. 2014. Pharmacokinetic and pharmacodynamic modeling in anesthetic field. Anesth Pain Med. 9:77–86.Hill AV. 1910. The possible effects of the aggregation of the molecules of haemoglobin on its dissociation curves. J Physiol. 40:4–7.Crommelin DJA., Sindelar RD., Meibohm B. 2019. Pharmaceutical biotechnology: fundamentals and applications. . 5th ed. Springer;Cham: DOI: 10.1007/978-3-030-00710-2.Sheiner LB., Stanski DR., Vozeh S., Miller RD., Ham J. 1979. Simultaneous modeling of pharmacokinetics and pharmacodynamics: application to d-tubocurarine. Clin Pharmacol Ther. 25:358–71. DOI: 10.1002/cpt1979253358. PMID: 761446.Holford NH., Sheiner LB. 1981. Pharmacokinetic and pharmacodynamic modeling in vivo. Crit Rev Bioeng. 5:273–322. PMID: 7023829.Segre G. 1968. Kinetics of interaction between drugs and biological systems. Farmaco Sci. 23:907–18. PMID: 5712792.Kowalski KG., Karim A. 1995. A semicompartmental modeling approach for pharmacodynamic data assessment. J Pharmacokinet Biopharm. 23:307–22. DOI: 10.1007/BF02354287. PMID: 8834198.Choi BM., Koh EH., Kim MG., Kim SH., Ok SY., Noh GJ. 2013. Temporal linear mode complexity as a surrogate measure of the anesthetic drug effects during sevoflurane anesthesia. Korean J Anesthesiol. 65:385–96. DOI: 10.4097/kjae.2013.65.5.385. PMID: 24363840. PMCID: PMC3866333.Bruhn J., Röpcke H., Hoeft A. 2000. Approximate entropy as an electroencephalographic measure of anesthetic drug effect during desflurane anesthesia. Anesthesiology. 92:715–26. DOI: 10.1097/00000542-200003000-00016. PMID: 10719951.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Population Pharmacokinetic and Pharmacodynamic Models of Propofol in Healthy Volunteers using NONMEM and Machine Learning Methods
- Neuromuscular blockade and pharmacokinetic-pharmacodynamic modeling
- Physiological spaces and multicompartmental pharmacokinetic models
- Obesity and anesthetic pharmacology: simulation of target-controlled infusion models of propofol and remifentanil
- Clinical application of intravenous anesthetic infusion with use of a pharmacokinetic-pharmacodynamic model in children