Allergy Asthma Immunol Res.
2018 Jan;10(1):88-94. 10.4168/aair.2018.10.1.88.
Real-Life Clinical Use of Symbicort® Maintenance and Reliever Therapy for Asthmatic Patients in Korea
- Affiliations
-
- 1Department of Internal Medicine, Eulji University School of Medicine, Daejeon, Korea.
- 2Department of Internal Medicine, Eulji University Hospital, Seoul, Korea.
- 3Department of Allergy and Clinical Immunology, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea.
- 4Division of Pulmonary Medicine and Allergy, Department of Internal Medicine, Hanyang University College of Medicine, Seoul, Korea. hjyoon@hanyang.ac.kr
- 5Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 6Department of Internal Medicine, Seoul Metropolitan Government-Seoul National University Boramae Medical Center, Seoul, Korea.
- 7Department of Internal Medicine, Inje University College of Medicine, Ilsan, Korea.
- 8Department of Internal Medicine, Dankook University College of Medicine, Cheonan, Korea.
- 9Medical Department, AstraZeneca Korea, Seoul, Korea.
- KMID: 2428854
- DOI: http://doi.org/10.4168/aair.2018.10.1.88
Abstract
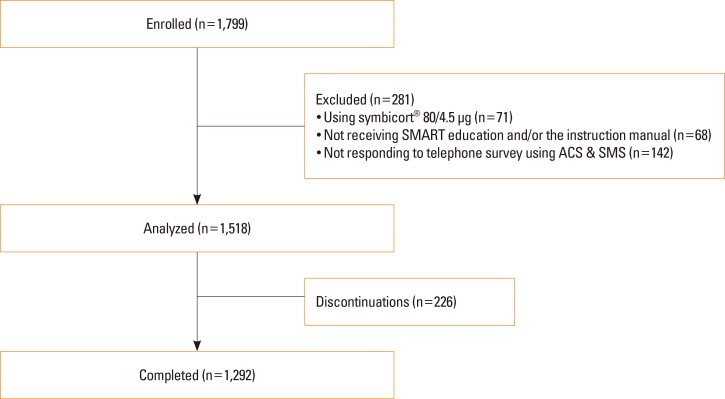
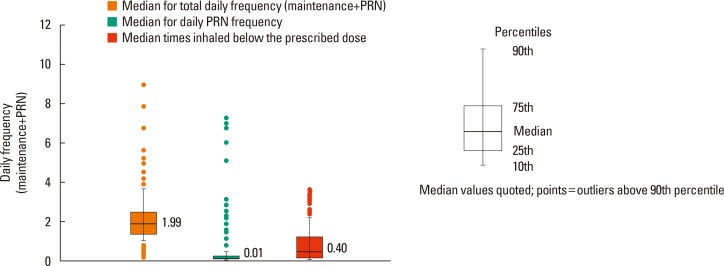
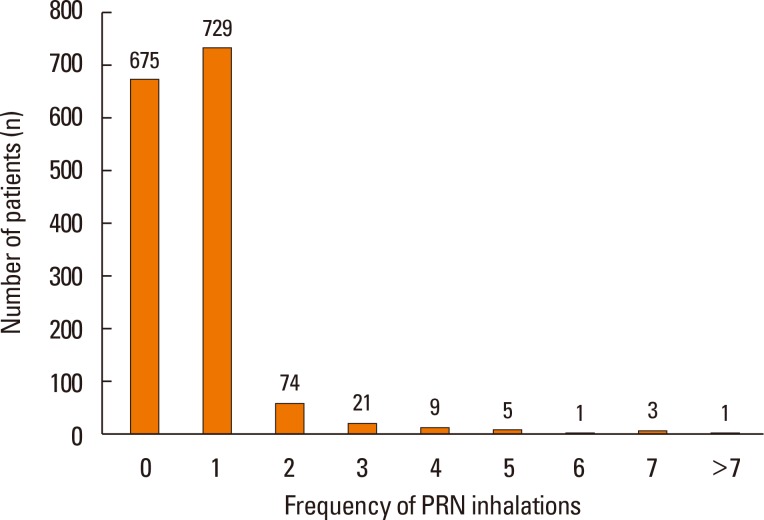
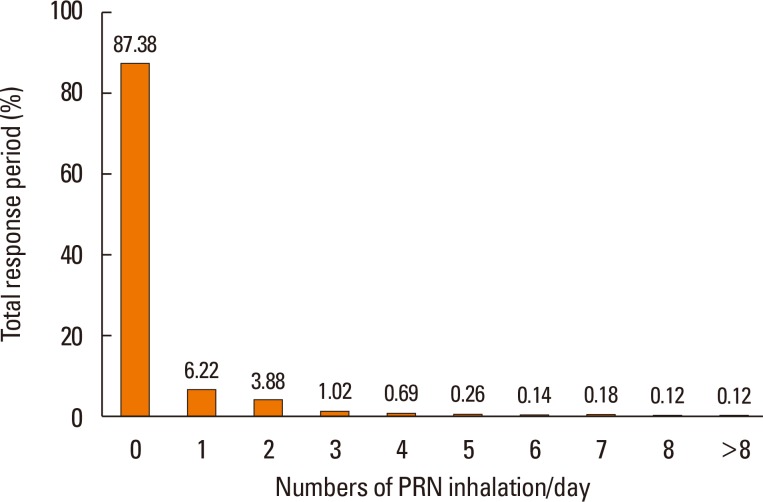
- The aim of this study was to examine the daily practice patterns of Symbicort® Maintenance and Reliever Therapy (SMART) in Korean asthmatic patients and to analyze clinical signs related to overuse. This study used an observational, multicenter, noninterventional, prospective, uncontrolled design for examining asthmatic patients prescribed SMART to assess the frequency and pattern of Symbicort® usage as a maintenance and reliever medication. The characteristics of patients showing signs of overuse (frequency of inhalation: 8 or more times per day) were also analyzed. Among the 1,518 patients analyzed, 1,292 (85.1%) completed the trial. The number of mean inhalations per day was 2.14±1.15; the number of patients who had at least 1 as needed usage (PRN) inhalation per day was 843 (55.5%); the mean frequency of PRN use was 0.25±0.67 inhalations per day. The number of patients who overused for at least 1 day was 260 (17.1%). In particular, young patients, patients with limited physical activity, and patients with nocturnal symptoms demonstrated high frequency of overuse. The frequency of overuse during SMART was not high in Korean asthmatic patients and the asthma status of follow-up outpatients improved overall. However, there is a need for careful education targeted toward younger patients, patients with limited physical activity, and patients with nocturnal symptoms owing to their tendency to frequently overuse.
Keyword
MeSH Terms
Figure
Reference
-
1. O'Byrne PM, Bisgaard H, Godard PP, Pistolesi M, Palmqvist M, Zhu Y, et al. Budesonide/formoterol combination therapy as both maintenance and reliever medication in asthma. Am J Respir Crit Care Med. 2005; 171:129–136. PMID: 15502112.2. Bousquet J, Boulet LP, Peters MJ, Magnussen H, Quiralte J, Martinez-Aguilar NE, et al. Budesonide/formoterol for maintenance and relief in uncontrolled asthma vs. high-dose salmeterol/fluticasone. Respir Med. 2007; 101:2437–2446. PMID: 17905575.
Article3. Kuna P, Peters MJ, Manjra AI, Jorup C, Naya IP, Martínez-Jimenez NE, et al. Effect of budesonide/formoterol maintenance and reliever therapy on asthma exacerbations. Int J Clin Pract. 2007; 61:725–736. PMID: 17362472.
Article4. Rabe KF, Pizzichini E, Ställberg B, Romero S, Balanzat AM, Atienza T, et al. Budesonide/formoterol in a single inhaler for maintenance and relief in mild-to-moderate asthma: a randomized, double-blind trial. Chest. 2006; 129:246–256. PMID: 16478838.5. Rabe KF, Atienza T, Magyar P, Larsson P, Jorup C, Lalloo UG. Effect of budesonide in combination with formoterol for reliever therapy in asthma exacerbations: a randomised controlled, double-blind study. Lancet. 2006; 368:744–753. PMID: 16935685.
Article6. Park HW, Tantisira KG. Genetic signatures of asthma exacerbation. Allergy Asthma Immunol Res. 2017; 9:191–199. PMID: 28293925.
Article7. Barnes PJ. Scientific rationale for using a single inhaler for asthma control. Eur Respir J. 2007; 29:587–595. PMID: 17329493.
Article8. Global Initiative for Asthma. Global strategy for asthma management and prevention 2016 [Internet]. [place unknown]: Global Initiative for Asthma;2016. cited 2016 Nov 8. Available from: http://www.ginasthma.org.9. Sears MR, Radner F. Safety of budesonide/formoterol maintenance and reliever therapy in asthma trials. Respir Med. 2009; 103:1960–1968. PMID: 19815402.
Article10. Cates CJ, Lasserson TJ. Regular treatment with formoterol and an inhaled corticosteroid versus regular treatment with salmeterol and an inhaled corticosteroid for chronic asthma: serious adverse events. Cochrane Database Syst Rev. 2010; CD007694. PMID: 20091646.
Article11. Patel M, Pilcher J, Pritchard A, Perrin K, Travers J, Shaw D, et al. Efficacy and safety of maintenance and reliever combination budesonide-formoterol inhaler in patients with asthma at risk of severe exacerbations: a randomised controlled trial. Lancet Respir Med. 2013; 1:32–42. PMID: 24321802.
Article12. Ställberg B, Naya I, Ekelund J, Eckerwall G. Real-life use of budesonide/formoterol in clinical practice: a 12-month follow-up assessment in a multi-national study of asthma patients established on single-inhaler maintenance and reliever therapy. Int J Clin Pharmacol Ther. 2015; 53:447–455. PMID: 25907171.
Article13. Ministry of Food and Drug Safety (KR). The Symbicort® prescribing information [Internet]. Cheongju: Ministry of Food and Drug Safety;cited 2016 Nov 8. Available from: https://ezdrug.mfds.go.kr/.14. Buhl R, Kuna P, Peters MJ, Andersson TL, Naya IP, Peterson S, et al. The effect of budesonide/formoterol maintenance and reliever therapy on the risk of severe asthma exacerbations following episodes of high reliever use: an exploratory analysis of two randomised, controlled studies with comparisons to standard therapy. Respir Res. 2012; 13:59. PMID: 22816878.
Article15. Vogelmeier C, D'Urzo A, Pauwels R, Merino JM, Jaspal M, Boutet S, et al. Budesonide/formoterol maintenance and reliever therapy: an effective asthma treatment option? Eur Respir J. 2005; 26:819–828. PMID: 16264042.16. Søes-Petersen U, Kava T, Dahle R, Lei Y, Dam N. Budesonide/formoterol maintenance and reliever therapy versus conventional best standard treatment in asthma in an attempted ‘real life’ setting. Clin Respir J. 2011; 5:173–182. PMID: 21679353.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Survey on the Current Status of Asthma Maintenance Therapy and the Impact of Asthma on Children and Family Life
- Update on the Evidence Regarding Maintenance Therapy
- HRCT Findings of Asthmatic Children under Maintenance Therapy
- Blood Metal Level’s Effects on Quality of Life of Aircraft Maintenance Workers
- Effectiveness of Same Versus Mixed Asthma Inhaler Devices: A Retrospective Observational Study in Primary Care