Allergy Asthma Immunol Res.
2012 Jul;4(4):184-191. 10.4168/aair.2012.4.4.184.
Effectiveness of Same Versus Mixed Asthma Inhaler Devices: A Retrospective Observational Study in Primary Care
- Affiliations
-
- 1Centre of Academic Primary Care, University of Aberdeen, Aberdeen, UK. david@rirl.org
- 2Research in Real Life Ltd, Cambridge, UK.
- 3School of Applied Sciences, University of Huddersfield, West Yorkshire, UK.
- 4Family Physician Airways Group of Canada, Richmond Hill, ON, Canada.
- 5Son Pisa Primary Health Care Centre, Balearic Health Service, Palma de Mallorca, Spain.
- 6Department of General Practice, University Medical Center Groningen, University of Groningen, Groningen, the Netherland.
- KMID: 1970706
- DOI: http://doi.org/10.4168/aair.2012.4.4.184
Abstract
- PURPOSE
Correct use of inhaler devices is fundamental to effective asthma management but represents an important challenge for patients. The correct inhalation manoeuvre differs markedly for different inhaler types. The objective of this study was to compare outcomes for patients prescribed the same inhaler device versus mixed device types for asthma controller and reliever therapy.
METHODS
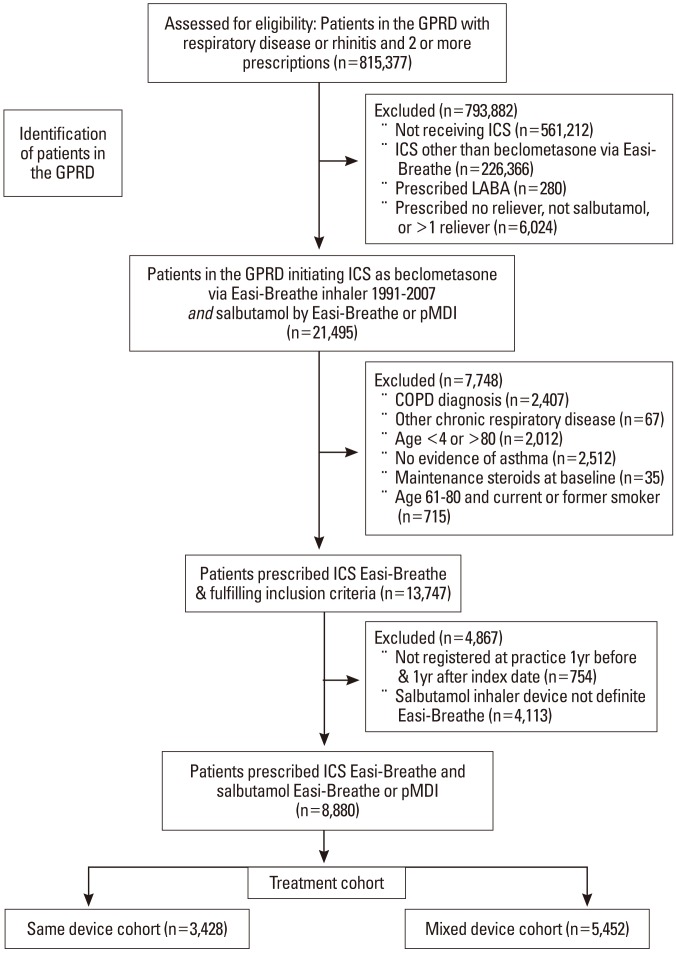
This retrospective observational study identified patients with asthma (ages 4-80 years) in a large primary care database who were prescribed an inhaled corticosteroid (ICS) for the first time. We compared outcomes for patients prescribed the same breath-actuated inhaler (BAI) for ICS controller and salbutamol reliever versus mixed devices (BAI for controller and pressurised metered-dose inhaler [pMDI] for reliever). The 2-year study included 1 baseline year before the ICS prescription (to identify and correct for confounding factors) and 1 outcome year. Endpoints were asthma control (defined as no hospital attendance for asthma, oral corticosteroids, or antibiotics for lower respiratory tract infection) and severe exacerbations (hospitalisation or oral corticosteroids for asthma).
RESULTS
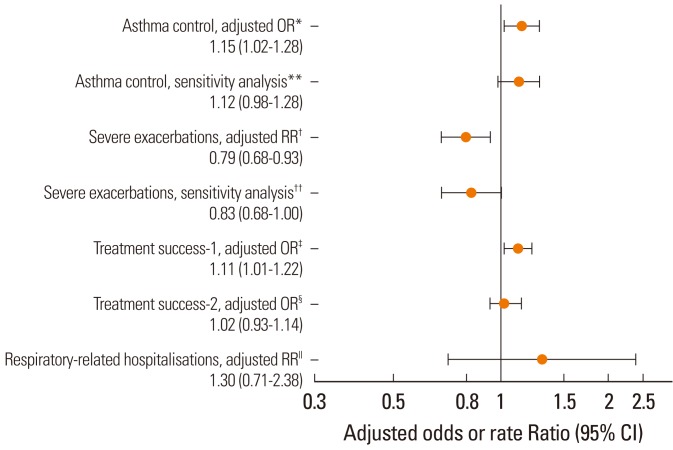
Patients prescribed the same device (n=3,428) were significantly more likely to achieve asthma control (adjusted odds ratio, 1.15; 95% confidence interval [CI], 1.02-1.28) and recorded significantly lower severe exacerbation rates (adjusted rate ratio, 0.79; 95% CI, 0.68-0.93) than those prescribed mixed devices (n=5,452).
CONCLUSIONS
These findings suggest that, when possible, the same device should be prescribed for both ICS and reliever therapy when patients are initiating ICS.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Pharmacotherapy in the management of asthma in the elderly: a review of clinical studies
Mi-Yeong Kim, Woo-Jung Song, Sang-Heon Cho
Asia Pac Allergy. 2016;6(1):3-15. doi: 10.5415/apallergy.2016.6.1.3.
Reference
-
1. Melani AS, Bonavia M, Cilenti V, Cinti C, Lodi M, Martucci P, Serra M, Scichilone N, Sestini P, Aliani M, Neri M. Gruppo Educazionale Associazione Italiana Pneumologi Ospedalieri. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med. 2011; 105:930–938. PMID: 21367593.
Article2. Molimard M, Raherison C, Lignot S, Depont F, Abouelfath A, Moore N. Assessment of handling of inhaler devices in real life: an observational study in 3811 patients in primary care. J Aerosol Med. 2003; 16:249–254. PMID: 14572322.
Article3. Sestini P, Cappiello V, Aliani M, Martucci P, Sena A, Vaghi A, Canessa PA, Neri M, Melani AS. Associazione Italiana Pneumologi Ospedalieri Educational Group. Prescription bias and factors associated with improper use of inhalers. J Aerosol Med. 2006; 19:127–136. PMID: 16796537.
Article4. Hardwell A, Barber V, Hargadon T, McKnight E, Holmes J, Levy ML. Technique training does not improve the ability of most patients to use pressurised metered-dose inhalers (pMDIs). Prim Care Respir J. 2011; 20:92–96. PMID: 21225221.
Article5. Giraud V, Roche N. Misuse of corticosteroid metered-dose inhaler is associated with decreased asthma stability. Eur Respir J. 2002; 19:246–251. PMID: 11866004.
Article6. Chrystyn H, Price D. Not all asthma inhalers are the same: factors to consider when prescribing an inhaler. Prim Care Respir J. 2009; 18:243–249. PMID: 19513494.
Article7. Broeders ME, Sanchis J, Levy ML, Crompton GK, Dekhuijzen PN. ADMIT Working Group. The ADMIT series--issues in inhalation therapy. 2. Improving technique and clinical effectiveness. Prim Care Respir J. 2009; 18:76–82. PMID: 19475324.8. Virchow JC, Crompton GK, Dal Negro R, Pedersen S, Magnan A, Seidenberg J, Barnes PJ. Importance of inhaler devices in the management of airway disease. Respir Med. 2008; 102:10–19. PMID: 17923402.
Article9. Haughney J, Price D, Barnes NC, Virchow JC, Roche N, Chrystyn H. Choosing inhaler devices for people with asthma: current knowledge and outstanding research needs. Respir Med. 2010; 104:1237–1245. PMID: 20472415.
Article10. Dolovich MB, Ahrens RC, Hess DR, Anderson P, Dhand R, Rau JL, Smaldone GC, Guyatt G. American College of Chest Physicians. American College of Asthma, Allergy, and Immunology. Device selection and outcomes of aerosol therapy: Evidence-based guidelines: American College of Chest Physicians/American College of Asthma, Allergy, and Immunology. Chest. 2005; 127:335–371. PMID: 15654001.11. Haughney J, Price D, Kaplan A, Chrystyn H, Horne R, May N, Moffat M, Versnel J, Shanahan ER, Hillyer EV, Tunsäter A, Bjermer L. Achieving asthma control in practice: understanding the reasons for poor control. Respir Med. 2008; 102:1681–1693. PMID: 18815019.
Article12. van der Palen J, Klein JJ, van Herwaarden CL, Zielhuis GA, Seydel ER. Multiple inhalers confuse asthma patients. Eur Respir J. 1999; 14:1034–1037. PMID: 10596686.
Article13. The Medicines and Healthcare Products Regulatory Agency [Internet]. General Practice Research Database. cited 2011 Oct 25. London: Available from: http://www.gprd.com/.14. Walley T, Mantgani A. The UK General Practice Research Database. Lancet. 1997; 350:1097–1099. PMID: 10213569.
Article15. Hansell A, Hollowell J, Nichols T, McNiece R, Strachan D. Use of the General Practice Research Database (GPRD) for respiratory epidemiology: a comparison with the 4th Morbidity Survey in General Practice (MSGP4). Thorax. 1999; 54:413–419. PMID: 10212105.
Article16. Petersen I, Johnson AM, Islam A, Duckworth G, Livermore DM, Hayward AC. Protective effect of antibiotics against serious complications of common respiratory tract infections: retrospective cohort study with the UK General Practice Research Database. BMJ. 2007; 335:982. PMID: 17947744.
Article17. Thomas M, von Ziegenweidt J, Lee AJ, Price D. High-dose inhaled corticosteroids versus add-on long-acting beta-agonists in asthma: an observational study. J Allergy Clin Immunol. 2009; 123:116–121.e10. PMID: 18986690.18. Price D, Martin RJ, Barnes N, Dorinsky P, Israel E, Roche N, Chisholm A, Hillyer EV, Kemp L, Lee AJ, von Ziegenweidt J, Colice G. Prescribing practices and asthma control with hydrofluoroalkane-beclomethasone and fluticasone: a real-world observational study. J Allergy Clin Immunol. 2010; 126:511–518.e1-10. PMID: 20692026.19. Reddel HK, Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, Casale TB, Chanez P, Enright PL, Gibson PG, de Jongste JC, Kerstjens HA, Lazarus SC, Levy ML, O'Byrne PM, Partridge MR, Pavord ID, Sears MR, Sterk PJ, Stoloff SW, Sullivan SD, Szefler SJ, Thomas MD, Wenzel SE. American Thoracic Society/European Respiratory Society Task Force on Asthma Control and Exacerbations. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009; 180:59–99. PMID: 19535666.20. Research in Real Life: Standard Operating Procedures [Internet]. cited 2011 Dec 17. Available from: http://www.optimumpatientcare.org/Docs/SOP%20Observational%20Database%20Studies.pdf.21. Dekhuijzen PN, Magnan A, Kneussl M. ADMIT Working Group. The ADMIT series - issues in inhalation therapy. 1) The goals of asthma treatment: can they be achieved? Prim Care Respir J. 2007; 16:341–348. PMID: 18066479.
Article22. Papi A, Haughney J, Virchow JC, Roche N, Palkonen S, Price D. Inhaler devices for asthma: a call for action in a neglected field. Eur Respir J. 2011; 37:982–985. PMID: 21532013.
Article23. Thomas M, Price D, Chrystyn H, Lloyd A, Williams AE, von Ziegenweidt J. Inhaled corticosteroids for asthma: impact of practice level device switching on asthma control. BMC Pulm Med. 2009; 9:1. PMID: 19121204.
Article24. Kozyrskyj AL, Dahl ME, Ungar WJ, Becker AB, Law BJ. Antibiotic treatment of wheezing in children with asthma: what is the practice? Pediatrics. 2006; 117:e1104–e1110. PMID: 16740813.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhalation medications in chronic airway disease
- Continued Innovation in Respiratory Care: The Importance of Inhaler Devices
- The Evaluation of an Education Program for Using an Inhaler Devices in Childhood Asthma
- Principles of the use of inhaler devices in asthma treatment
- Comparing Inhaler Use Technique Based on Inhaler Type in Elderly Patients with Respiratory Disease