Yonsei Med J.
2017 Mar;58(2):282-289. 10.3349/ymj.2017.58.2.282.
A Thin Left Atrial Antral Wall Around the Pulmonary Vein Reflects Structural Remodeling by Atrial Fibrillation and is Associated with Stroke
- Affiliations
-
- 1Department of Cardiology, School of Medicine, Ewha Womans University, Seoul, Korea.
- 2Department of Radiology, Research Institute of Radiological Science, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Cardiology, Yonsei University Health System, Seoul, Korea. cby6908@yuhs.ac
- KMID: 2427115
- DOI: http://doi.org/10.3349/ymj.2017.58.2.282
Abstract
- PURPOSE
Circumferential pulmonary (PV) vein isolation (CPVI) is the most important treatment strategy for atrial fibrillation (AF). While understanding left atrial wall thickness around PVs (PVWT) prior to catheter ablation is important, its clinical implications are not known. This study aimed to evaluate PVWT characteristics according to underlying disease and to identify associations between PVWT and reconnections of PV potentials (PVPs) in redo ablation.
MATERIALS AND METHODS
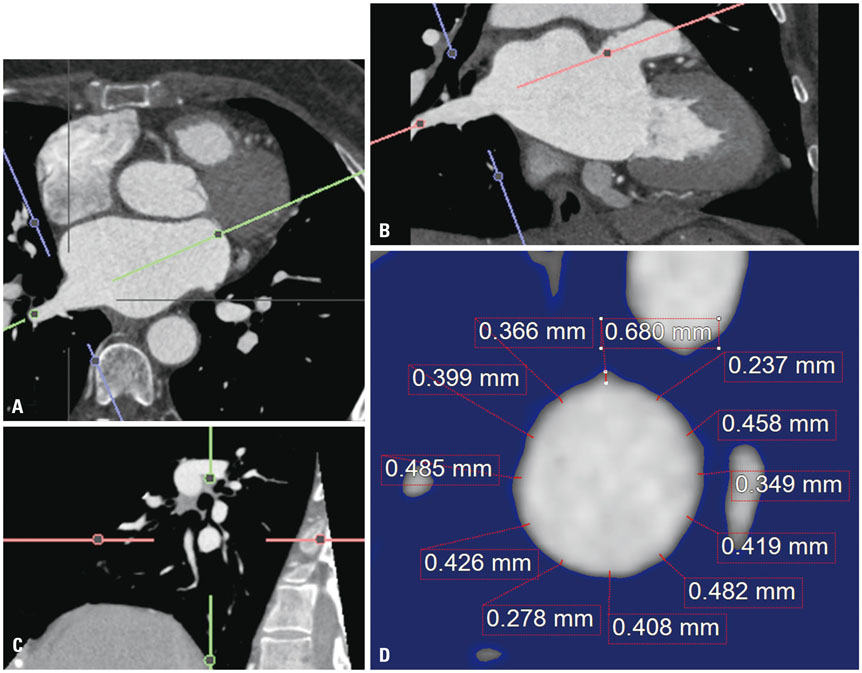
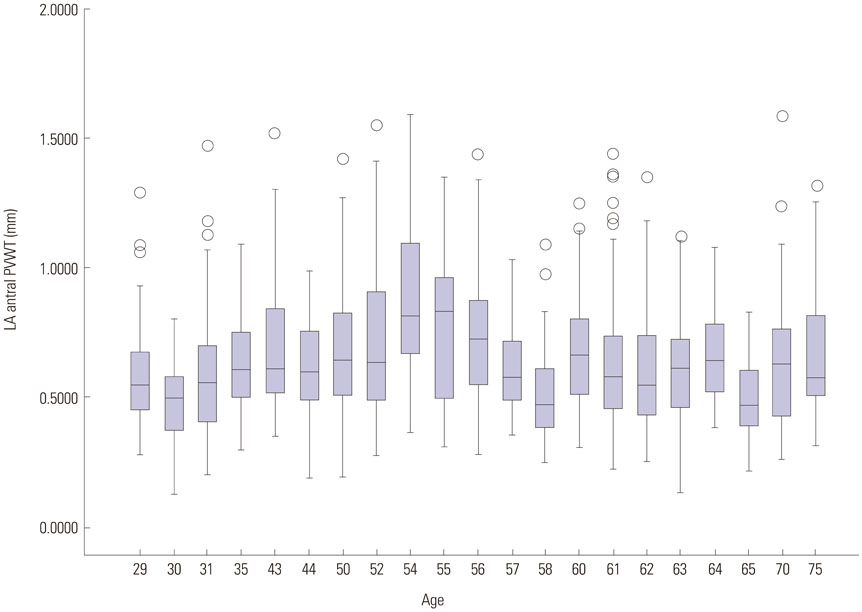
In 28 patients who underwent redo-AF ablation, PVWT and reconnected PVPs were evaluated at 12 sites (1-12 o'clock) around each PV. Clinical characteristics including stroke and CHAâ‚‚DSâ‚‚-VASc scores were analyzed according to the PVWT.
RESULTS
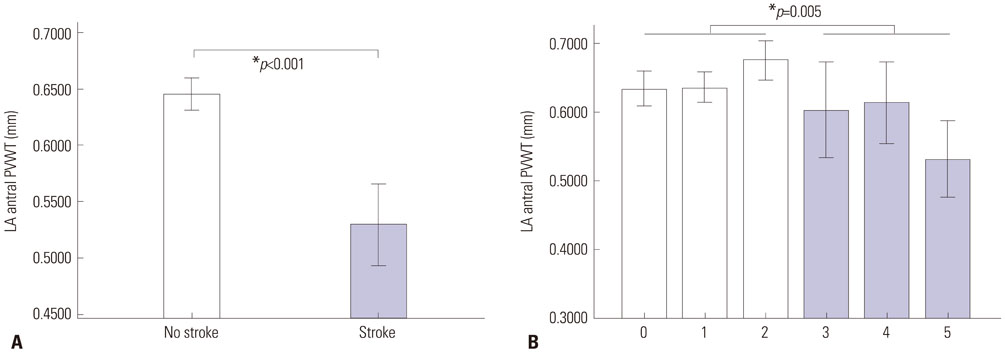
The PVWT was thicker in males than females (p<0.001) and in those with diabetes (p=0.045) or heart failure (p=0.002) than in those without. Patients with strokes or high CHA₂DS₂-VASc scores (≥3) had significantly thinner PVWTs than those without strokes or low CHA₂DS₂-VASc scores (p<0.001). In redo-ablation, reconnected PVPs were detected in 60 (53.6%) of 112 PVs, and the PVs were thicker (p<0.001) and had more reconnected PVs (p=0.009) than right PVs. A PVWT of >0.6 mm predicted PV reconnections with a sensitivity of 76.7% and specificity of 52.2% with an area under the curve of 0.695.
CONCLUSION
Thick PVWs were associated with diabetes and heart failure, and also showed significant inverse correlations with stroke and the CHAâ‚‚DSâ‚‚-VASc score. Thick PVWs were associated with reconnected PVPs after the CPVI, which were related to AF recurrence.
Keyword
MeSH Terms
Figure
Reference
-
1. Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998; 339:659–666.
Article2. Nademanee K, McKenzie J, Kosar E, Schwab M, Sunsaneewitayakul B, Vasavakul T, et al. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J Am Coll Cardiol. 2004; 43:2044–2053.
Article3. Verma A, Mantovan R, Macle L, De Martino G, Chen J, Morillo CA, et al. Substrate and trigger ablation for reduction of atrial fibrillation (STAR AF): a randomized, multicentre, international trial. Eur Heart J. 2010; 31:1344–1356.
Article4. Park J, Park CH, Lee HJ, Wi J, Uhm JS, Pak HN, et al. Left atrial wall thickness rather than epicardial fat thickness is related to complex fractionated atrial electrogram. Int J Cardiol. 2014; 172:e411–e413.
Article5. Wi J, Lee HJ, Uhm JS, Kim JY, Pak HN, Lee M, et al. Complex fractionated atrial electrograms related to left atrial wall thickness. J Cardiovasc Electrophysiol. 2014; 25:1141–1149.
Article6. Verma A, Jiang CY, Betts TR, Chen J, Deisenhofer I, Mantovan R, et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med. 2015; 372:1812–1822.
Article7. European Heart Rhythm Association (EHRA). European Cardiac Arrhythmia Scoiety (ECAS). American College of Cardiology (ACC). American Heart Association (AHA). Society of Thoracic Surgeons (STS). Calkins H, et al. HRS/EHRA/ECAS expert Consensus Statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2007; 4:816–861.
Article8. Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009; 22:107–133.
Article9. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005; 18:1440–1463.
Article10. Parkash R, Green MS, Kerr CR, Connolly SJ, Klein GJ, Sheldon R, et al. The association of left atrial size and occurrence of atrial fibrillation: a prospective cohort study from the Canadian Registry of Atrial Fibrillation. Am Heart J. 2004; 148:649–654.
Article11. Reant P, Lafitte S, Jaïs P, Serri K, Weerasooriya R, Hocini M, et al. Reverse remodeling of the left cardiac chambers after catheter ablation after 1 year in a series of patients with isolated atrial fibrillation. Circulation. 2005; 112:2896–2903.
Article12. Beukema WP, Elvan A, Sie HT, Misier AR, Wellens HJ. Successful radiofrequency ablation in patients with previous atrial fibrillation results in a significant decrease in left atrial size. Circulation. 2005; 112:2089–2095.
Article13. Park JH, Pak HN, Choi EJ, Jang JK, Kim SK, Choi DH, et al. The relationship between endocardial voltage and regional volume in electroanatomical remodeled left atria in patients with atrial fibrillation: comparison of three-dimensional computed tomographic images and voltage mapping. J Cardiovasc Electrophysiol. 2009; 20:1349–1356.
Article14. Park MJ, Jung JI, Oh YS, Youn HJ. Assessment of the structural remodeling of the left atrium by 64-multislice cardiac CT: comparative studies in controls and patients with atrial fibrillation. Int J Cardiol. 2012; 159:181–186.
Article15. Avelar E, Durst R, Rosito GA, Thangaroopan M, Kumar S, Tournoux F, et al. Comparison of the accuracy of multidetector computed tomography versus two-dimensional echocardiography to measure left atrial volume. Am J Cardiol. 2010; 106:104–109.
Article16. Heo R, Hong GR, Kim YJ, Mancina J, Cho IJ, Shim CY, et al. Automated quantification of left atrial size using three-beat averaging real-time three dimensional Echocardiography in patients with atrial fibrillation. Cardiovasc Ultrasound. 2015; 13:38.
Article17. Haines D. Biophysics of ablation: application to technology. J Cardiovasc Electrophysiol. 2004; 15:10 Suppl. S2–S11.
Article18. van Hoeven KH, Factor SM. A comparison of the pathological spectrum of hypertensive, diabetic, and hypertensive-diabetic heart disease. Circulation. 1990; 82:848–855.
Article19. van Heerebeek L, Hamdani N, Handoko ML, Falcao-Pires I, Musters RJ, Kupreishvili K, et al. Diastolic stiffness of the failing diabetic heart: importance of fibrosis, advanced glycation end products, and myocyte resting tension. Circulation. 2008; 117:43–51.20. Fischer VW, Barner HB, Leskiw ML. Capillary basal laminar thichness in diabetic human myocardium. Diabetes. 1979; 28:713–719.
Article21. Devereux RB, Roman MJ, Paranicas M, O'Grady MJ, Lee ET, Welty TK, et al. Impact of diabetes on cardiac structure and function: the strong heart study. Circulation. 2000; 101:2271–2276.
Article22. Nakamura K, Funabashi N, Uehara M, Ueda M, Murayama T, Takaoka H, et al. Left atrial wall thickness in paroxysmal atrial fibrillation by multislice-CT is initial marker of structural remodeling and predictor of transition from paroxysmal to chronic form. Int J Cardiol. 2011; 148:139–147.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Controlled Atrial Fibrillation after Pulmonary Vein Stenting
- Atrial Fibrillation in a Patient with Left Ventricular Hypertrophy after Induction of General Anesthesia: A case report
- The Mechanism of and Preventive Therapy for Stroke in Patients with Atrial Fibrillation
- Cardioembolic Stroke in Atrial Fibrillation-Rationale for Preventive Closure of the Left Atrial Appendage
- Role of non‑pulmonary vein triggers in persistent atrial fibrillation