Allergy Asthma Immunol Res.
2019 Jan;11(1):55-67. 10.4168/aair.2019.11.1.55.
Adaptation and Validation of the Korean Version of the Urticaria Control Test and Its Correlation With Salivary Cortisone
- Affiliations
-
- 1Department of Internal Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 2Institute of Laboratory Medicine, Clinical Chemistry and Molecular Diagnostics, University of Leipzig, Leipzig, Germany.
- 3Department of Allergy and Clinical Immunology, Ajou University School of Medicine, Suwon, Korea. ye9007@ajou.ac.kr
- 4Clinical Trial Center, Ajou University Medical Center, Suwon, Korea.
- 5Department of Pulmonary, Allergy and Critical Care Medicine, Kangdong Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea.
- KMID: 2426785
- DOI: http://doi.org/10.4168/aair.2019.11.1.55
Abstract
- PURPOSE
Frequent changes in chronic urticaria (CU) activity over time can cause psychological stress, which also serves as a trigger of CU. To measure the control status of CU, the Urticaria Control Test (UCT) was developed in Germany. This study aimed to investigate the validity, reliability and responsiveness to changes in CU for the Korean version of the UCT (K-UCT) and its relation with salivary cortisol and cortisone levels.
METHODS
Linguistic adaptation of the UCT into Korean was conducted. A total of 96 CU patients were enrolled, and 80 of them completed the study. The K-UCT and other outcome scores for CU were measured and repeated after 4 weeks of treatment. Control status was classified by physicians into well-controlled, partly-controlled, and uncontrolled CU. Salivary cortisol and cortisone were measured by liquid chromatography-tandem mass spectrometry.
RESULTS
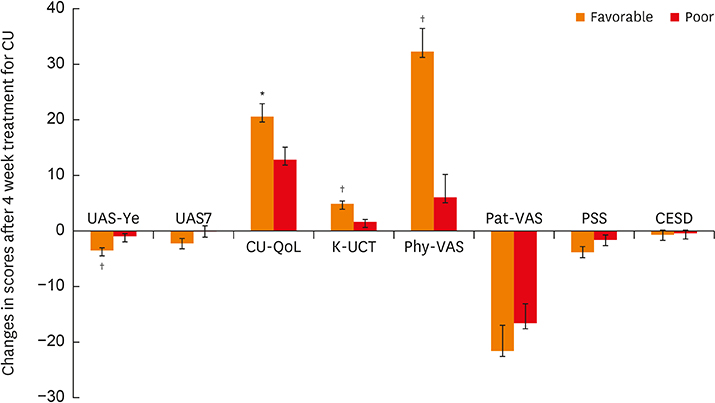
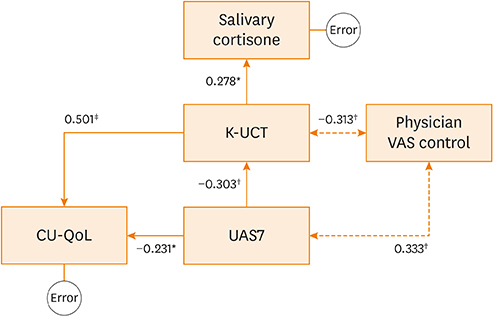
Excellent internal consistency and intra-class reliability were obtained. Strong correlations between the K-UCT and disease severity, reflected in the Urticaria Activity Score (UAS)/global assessment of urticaria control by physicians/patient assessment of symptom severity/CU-specific quality of life were noted. K-UCT scores ≥12 were found to be optimal for determining well-controlled CU (sensitivity, 75.0%; specificity, 758%; area under the curve, 0.824). Perceived stress scale scores were significantly correlated with the UAS and the K-UCT. Salivary cortisone levels were significantly correlated with K-UCT (r = 0.308, P = 0.009) and differed significantly according to control status determined by a K-UCT ≥12.
CONCLUSIONS
This study demonstrated that the K-UCT can be a valid instrument with which to gauge CU control status in Korean patients. Further studies are needed to validate salivary cortisone as a biomarker for CU control.
MeSH Terms
Figure
Reference
-
1. Zuberbier T, Aberer W, Asero R, Bindslev-Jensen C, Brzoza Z, Canonica GW, et al. The EAACI/GA2LEN/EDF/WAO Guideline for the definition, classification, diagnosis, and management of urticaria: the 2013 revision and update. Allergy. 2014; 69:868–887.2. O'Donnell BF, Lawlor F, Simpson J, Morgan M, Greaves MW. The impact of chronic urticaria on the quality of life. Br J Dermatol. 1997; 136:197–201.3. Zuberbier T, Balke M, Worm M, Edenharter G, Maurer M. Epidemiology of urticaria: a representative cross-sectional population survey. Clin Exp Dermatol. 2010; 35:869–873.
Article4. Lee N, Lee JD, Lee HY, Kang DR, Ye YM. Epidemiology of chronic urticaria in Korea using the Korean health insurance database, 2010–2014. Allergy Asthma Immunol Res. 2017; 9:438–445.
Article5. Weller K, Groffik A, Church MK, Hawro T, Krause K, Metz M, et al. Development and validation of the Urticaria Control Test: a patient-reported outcome instrument for assessing urticaria control. J Allergy Clin Immunol. 2014; 133:1365–1372. 1372.e1–1366.6. Yang HY, Sun CC, Wu YC, Wang JD. Stress, insomnia, and chronic idiopathic urticaria--a case-control study. J Formos Med Assoc. 2005; 104:254–263.7. Dyke SM, Carey BS, Kaminski ER. Effect of stress on basophil function in chronic idiopathic urticaria. Clin Exp Allergy. 2008; 38:86–92.
Article8. Hellhammer DH, Wüst S, Kudielka BM. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology. 2009; 34:163–171.
Article9. Smith RE, Maguire JA, Stein-Oakley AN, Sasano H, Takahashi K, Fukushima K, et al. Localization of 11 beta-hydroxysteroid dehydrogenase type II in human epithelial tissues. J Clin Endocrinol Metab. 1996; 81:3244–3248.
Article10. Bae YJ, Gaudl A, Jaeger S, Stadelmann S, Hiemisch A, Kiess W, et al. Immunoassay or LC-MS/MS for the measurement of salivary cortisol in children? Clin Chem Lab Med. 2016; 54:811–822.
Article11. Blair J, Adaway J, Keevil B, Ross R. Salivary cortisol and cortisone in the clinical setting. Curr Opin Endocrinol Diabetes Obes. 2017; 24:161–168.
Article12. Konstantinou GN, Asero R, Maurer M, Sabroe RA, Schmid-Grendelmeier P, Grattan CE. EAACI/GA2LEN task force consensus report: the autologous serum skin test in urticaria. Allergy. 2009; 64:1256–1268.13. Hawro T, Ohanyan T, Schoepke N, Metz M, Peveling-Oberhag A, Staubach P, et al. Comparison and interpretability of the available Urticaria Activity Scores. Allergy. 2018; 73:251–255.
Article14. Ye YM, Park JW, Kim SH, Choi JH, Hur GY, Lee HY, et al. Clinical evaluation of the computerized chronic urticaria-specific quality of life questionnaire in Korean patients with chronic urticaria. Clin Exp Dermatol. 2012; 37:722–728.
Article15. Lee EH, Chung BY, Suh CH, Jung JY. Korean versions of the Perceived Stress Scale (PSS-14, 10 and 4): psychometric evaluation in patients with chronic disease. Scand J Caring Sci. 2015; 29:183–192.
Article16. Barth J, de Boer WE, Busse JW, Hoving JL, Kedzia S, Couban R, et al. Inter-rater agreement in evaluation of disability: systematic review of reproducibility studies. BMJ. 2017; 356:j14.
Article17. Gaudl A, Kratzsch J, Bae YJ, Kiess W, Thiery J, Ceglarek U. Liquid chromatography quadrupole linear ion trap mass spectrometry for quantitative steroid hormone analysis in plasma, urine, saliva and hair. J Chromatogr A. 2016; 1464:64–71.
Article18. Soh SE, McGinley JL, Watts JJ, Iansek R, Murphy AT, Menz HB, et al. Determinants of health-related quality of life in people with Parkinson's disease: a path analysis. Qual Life Res. 2013; 22:1543–1553.
Article19. Trinh HK, Pham DL, Ban GY, Lee HY, Park HS, Ye YM. Altered systemic adipokines in patients with chronic urticaria. Int Arch Allergy Immunol. 2016; 171:102–110.
Article20. Baiardini I, Braido F, Bindslev-Jensen C, Bousquet PJ, Brzoza Z, Canonica GW, et al. Recommendations for assessing patient-reported outcomes and health-related quality of life in patients with urticaria: a GA2LEN taskforce position paper. Allergy. 2011; 66:840–844.21. Kulthanan K, Chularojanamontri L, Tuchinda P, Rujitharanawong C, Maurer M, Weller K. Validity, reliability and interpretability of the Thai version of the Urticaria Control Test (UCT). Health Qual Life Outcomes. 2016; 14:61.
Article22. García-Díez I, Curto-Barredo L, Weller K, Pujol RM, Maurer M, Giménez-Arnau AM. Cross-cultural adaptation of the Urticaria Control Test from German to Castilian Spanish. Actas Dermosifiliogr. 2015; 106:746–752.
Article23. Ohanyan T, Schoepke N, Bolukbasi B, Metz M, Hawro T, Zuberbier T, et al. Responsiveness and minimal important difference of the Urticaria Control Test. J Allergy Clin Immunol. 2017; 140:1710–1713.e11.
Article24. Lindsay K, Goulding J, Solomon M, Broom B. Treating chronic spontaneous urticaria using a brief ‘whole person’ treatment approach: a proof-of-concept study. Clin Transl Allergy. 2015; 5:40.
Article25. Hammen C, Kim EY, Eberhart NK, Brennan PA. Chronic and acute stress and the prediction of major depression in women. Depress Anxiety. 2009; 26:718–723.
Article26. Varghese R, Rajappa M, Chandrashekar L, Kattimani S, Archana M, Munisamy M, et al. Association among stress, hypocortisolism, systemic inflammation, and disease severity in chronic urticaria. Ann Allergy Asthma Immunol. 2016; 116:344–348.e1.
Article27. Kim JE, Cho BK, Cho DH, Park HJ. Expression of hypothalamic-pituitary-adrenal axis in common skin diseases: evidence of its association with stress-related disease activity. Acta Derm Venereol. 2013; 93:387–393.
Article28. Debono M, Harrison RF, Whitaker MJ, Eckland D, Arlt W, Keevil BG, et al. Salivary cortisone reflects cortisol exposure under physiological conditions and after hydrocortisone. J Clin Endocrinol Metab. 2016; 101:1469–1477.
Article29. Langelaan ML, Kisters JM, Oosterwerff MM, Boer AK. Salivary cortisol in the diagnosis of adrenal insufficiency: cost efficient and patient friendly. Endocr Connect. 2018; 7:560–566.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Music Therapy on Subjective Stress Response, Salivary Cortisol, and Fatigue for Intensive Care Nurses
- Influence of Epinephrine, Cortisone Acetate and Adrenocorticotrophic Hormone on Gastric Secretion
- Translation and Validation of a Korean Version of the Xerostomia Inventory in Patients with Primary Sjögren's Syndrome
- Influences of Anti-tuberculous Agents and Cortisone on Renal Tuberculosis Induced in Rabbit
- Cross-Cultural Adaptation and Validation of the Korean Version of the Neck Disability Index