Investig Clin Urol.
2016 Jul;57(4):249-259. 10.4111/icu.2016.57.4.249.
Progressive changes in detrusor function and micturition patterns with chroinc bladder ischemia
- Affiliations
-
- 1Department of Urology, VA Boston Healthcare System and Boston University School of Medicine, Boston, MA, USA. kazadzoi@bu.edu
- KMID: 2426502
- DOI: http://doi.org/10.4111/icu.2016.57.4.249
Abstract
- PURPOSE
Lower urinary tract symptoms (LUTS) are bothersome constellation of voiding symptoms in men and women as they age. Multiple factors and comorbidities are attributed to this problem but underlying mechanisms of nonobstructive nonneurogenic detrusor overactivity, detrusor underactivity and LUTS remain largely unknown. Our goal was to characterize detrusor function and voiding patterns in relation to muscarinic receptors expression, nerve fiber density, and neural ultrastructure in chronic bladder ischemia.
MATERIALS AND METHODS
Iliac artery atherosclerosis and bladder ischemia were produced in male Sprague-Dawley rats. At 8 and 16 weeks after ischemia, micturition patterns and cystometrograms were recorded in conscious rats then bladder blood flow and nonvoiding spontaneous contractions were measured under general anesthesia. Bladder tissues were processed for Western blotting, immunostaining, and transmission electron microscopy.
RESULTS
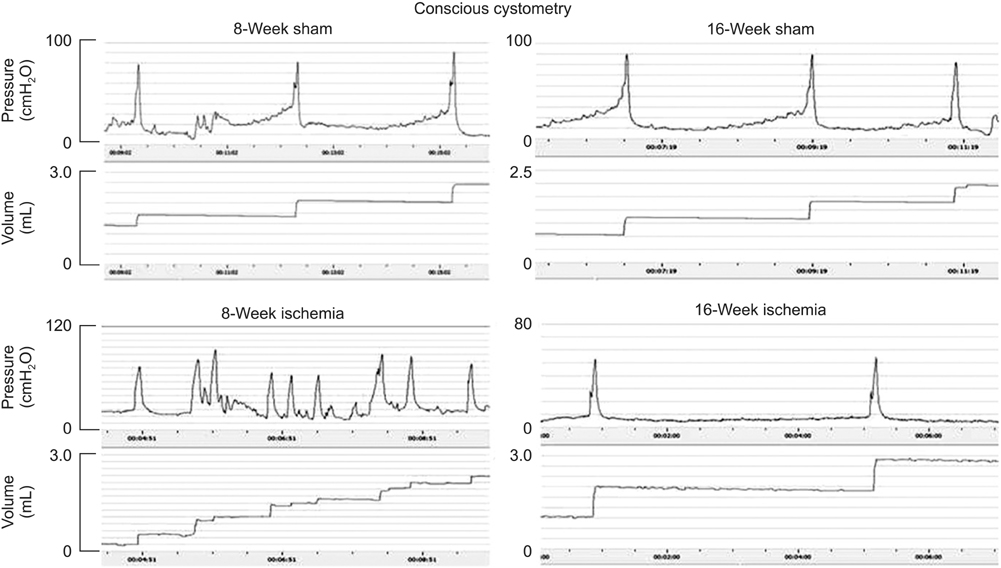
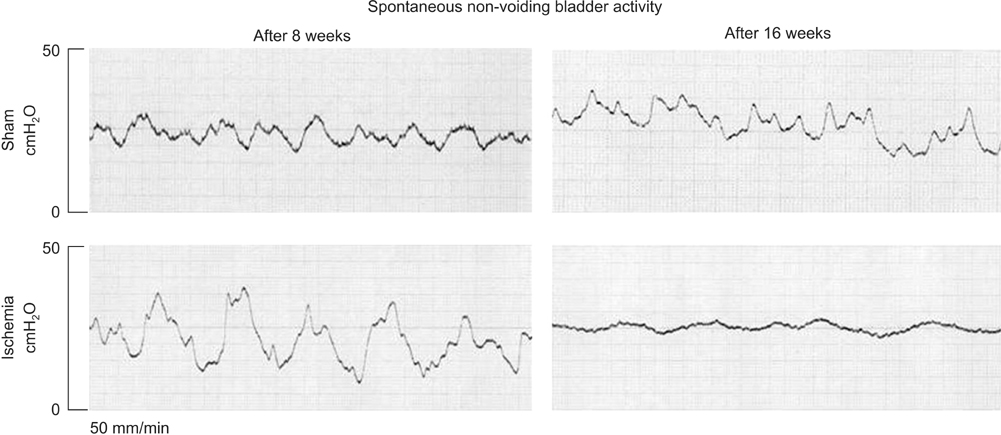
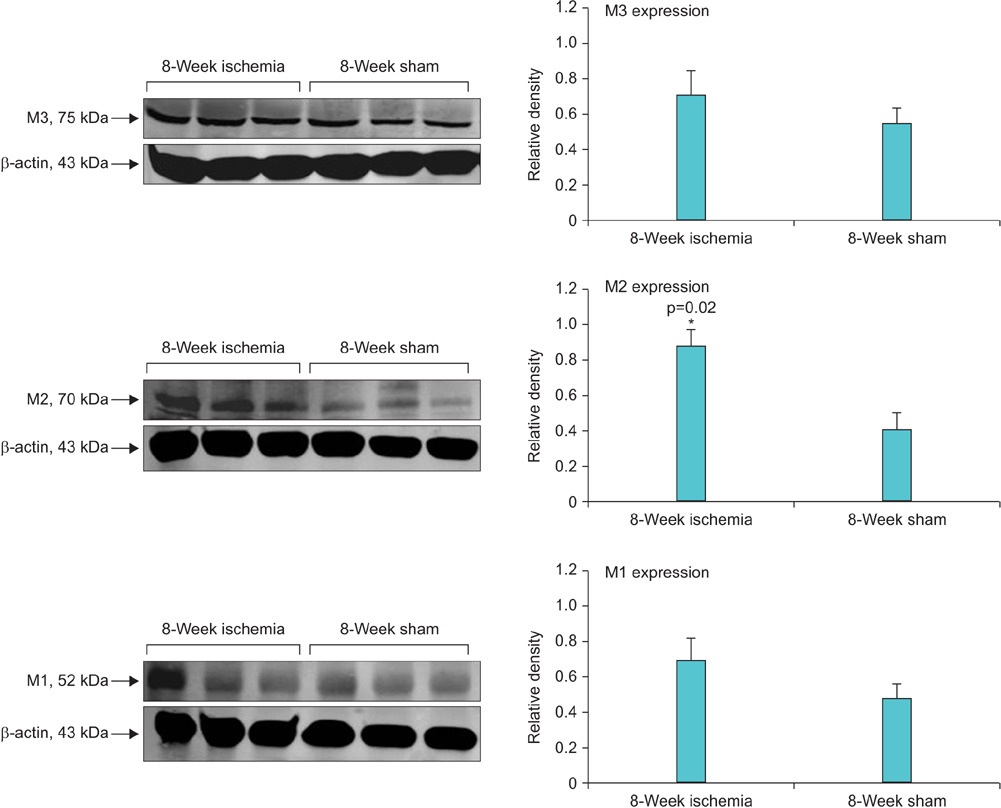
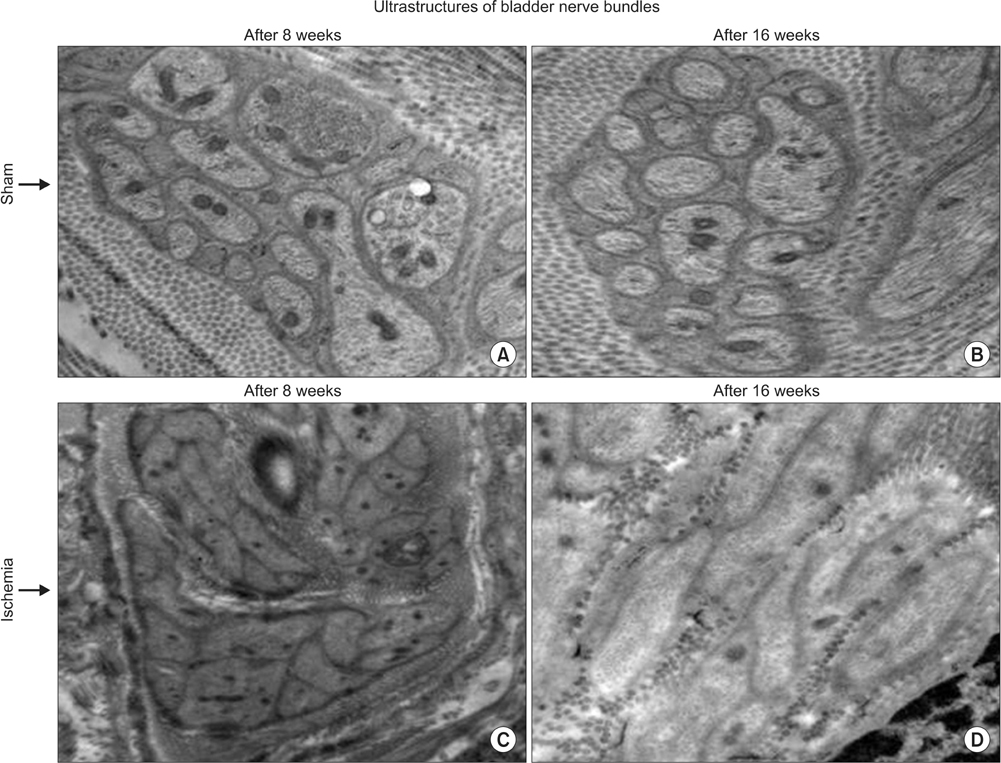
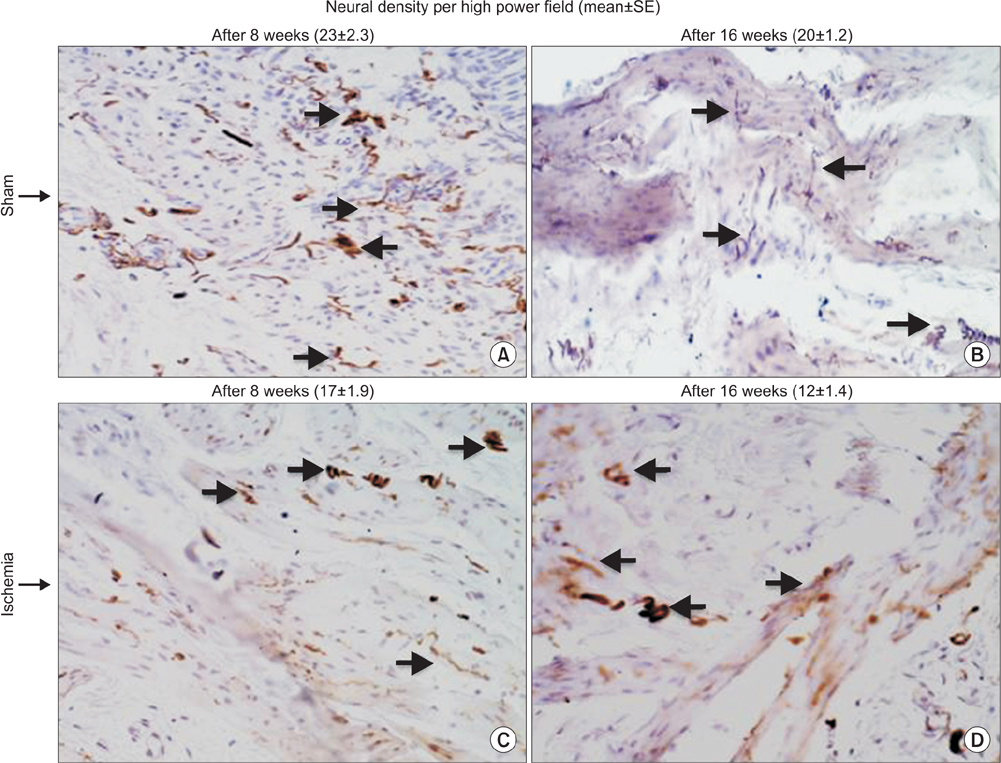
Bladder responses to ischemic insult depended on the duration of ischemia. Micturition patterns and cystometric changes at 8-week ischemia suggested detrusor overactivity, while voiding behavior and cystometrograms at 16-week ischemia implied abnormal detrusor function resembling underactivity. Upregulation of muscarinic M2 receptor was found after 8- and 16 weeks of ischemia. Downregulation of M3 and upregulation of M1 were detected at 16-week ischemia. Neural structural damage and marked neurodegeneration were found after 8 and 16 weeks of ischemia, respectively.
CONCLUSIONS
Prolonged ischemia may be a mediating variable in progression of overactive bladder to dysfunctional patterns similar to detrusor underactivity. The mechanism appears to involve differential expression of M1, M2, and M3 receptors, neural structural injury, and progressive loss of nerve fibers.
Keyword
MeSH Terms
-
Animals
Axons/ultrastructure
Chronic Disease
Disease Progression
Ischemia/metabolism/pathology/*physiopathology
Male
Microscopy, Electron
Muscle Contraction/physiology
Nerve Fibers/pathology
Rats, Sprague-Dawley
Receptors, Muscarinic/metabolism
Regional Blood Flow/physiology
Urinary Bladder/*blood supply/innervation/metabolism/physiopathology
Urinary Bladder, Overactive/metabolism/pathology/*physiopathology
Urination/*physiology
Figure
Cited by 1 articles
-
Pathophysiology of the underactive bladder
Naoki Aizawa, Yasuhiko Igawa
Investig Clin Urol. 2017;58(Suppl 2):S82-S89. doi: 10.4111/icu.2017.58.S2.S82.
Reference
-
1. Taylor JA 3rd, Kuchel GA. Detrusor underactivity: clinical features and pathogenesis of an underdiagnosed geriatric condition. J Am Geriatr Soc. 2006; 54:1920–1932.2. Hoag N, Gani J. Underactive bladder: clinical features, urodynamic parameters, and treatment. Int Neurourol J. 2015; 19:185–189.3. Sekido N, Kida J, Wakamatsu D, Okada H, Matsuya H. Does an "overactive to underactive bladder transition" phenomenon exist in a rat lumbar spinal canal stenosis model? Open J Urol. 2015; 5:57–64.4. Azadzoi KM, Tarcan T, Kozlowski R, Krane RJ, Siroky MB. Overactivity and structural changes in the chronically ischemic bladder. J Urol. 1999; 162:1768–1778.5. Nomiya M, Yamaguchi O, Akaihata H, Hata J, Sawada N, Kojima Y, et al. Progressive vascular damage may lead to bladder underactivity in rats. J Urol. 2014; 191:1462–1469.6. Azadzoi KM, Chen BG, Radisavljevic ZM, Siroky MB. Molecular reactions and ultrastructural damage in the chronically ischemic bladder. J Urol. 2011; 186:2115–2122.7. Nomiya M, Yamaguchi O, Andersson KE, Sagawa K, Aikawa K, Shishido K, et al. The effect of atherosclerosis-induced chronic bladder ischemia on bladder function in the rat. Neurourol Urodyn. 2012; 31:195–200.8. Pinggera GM, Mitterberger M, Steiner E, Pallwein L, Frauscher F, Aigner F, et al. Association of lower urinary tract symptoms and chronic ischaemia of the lower urinary tract in elderly women and men: assessment using colour Doppler ultrasonography. BJU Int. 2008; 102:470–474.9. De EJ, Hou P, Estrera AL, Sdringola S, Kramer LA, Graves DE, et al. Pelvic ischemia is measurable and symptomatic in patients with coronary artery disease: a novel application of dynamic contrast-enhanced magnetic resonance imaging. J Sex Med. 2008; 5:2635–2645.10. Pinggera GM, Mitterberger M, Pallwein L, Schuster A, Herwig R, Frauscher F, et al. alpha-Blockers improve chronic ischaemia of the lower urinary tract in patients with lower urinary tract symptoms. BJU Int. 2008; 101:319–324.11. Jeong SJ, Kim HJ, Lee YJ, Lee JK, Lee BK, Choo YM, et al. Prevalence and clinical features of detrusor underactivity among elderly with lower urinary tract symptoms: a comparison between men and women. Korean J Urol. 2012; 53:342–348.12. Abarbanel J, Marcus EL. Impaired detrusor contractility in community-dwelling elderly presenting with lower urinary tract symptoms. Urology. 2007; 69:436–440.13. Azadzoi KM, Radisavljevic ZM, Golabek T, Yalla SV, Siroky MB. Oxidative modification of mitochondrial integrity and nerve fiber density in the ischemic overactive bladder. J Urol. 2010; 183:362–369.14. Hegde SS, Choppin A, Bonhaus D, Briaud S, Loeb M, Moy TM, et al. Functional role of M2 and M3 muscarinic receptors in the urinary bladder of rats in vitro and in vivo. Br J Pharmacol. 1997; 120:1409–1418.15. Hegde SS, Eglen RM. Muscarinic receptor subtypes modulating smooth muscle contractility in the urinary bladder. Life Sci. 1999; 64:419–428.16. Yoshida M, Homma Y, Inadome A, Yono M, Seshita H, Miyamoto Y, et al. Age-related changes in cholinergic and purinergic neurotransmission in human isolated bladder smooth muscles. Exp Gerontol. 2001; 36:99–109.17. Ehlert FJ, Ostrom RS, Sawyer GW. Subtypes of the muscarinic receptor in smooth muscle. Life Sci. 1997; 61:1729–1740.18. Braverman AS, Wess J, Ruggieri MR. M-2 mediated bladder contractions require RHO kinase whereas M-3 receptors activate alternative, parallel pathways: findings from muscarinic receptor knock out mice. J Urol. 2006; 175:182.19. Braverman AS, Tibb AS, Ruggieri MR Sr. M2 and M3 muscarinic receptor activation of urinary bladder contractile signal transduction. I. Normal rat bladder. J Pharmacol Exp Ther. 2006; 316:869–874.20. Braverman AS, Luthin GR, Ruggieri MR. M2 muscarinic receptor contributes to contraction of the denervated rat urinary bladder. Am J Physiol. 1998; 275(5 Pt 2):R1654–R1660.21. Urrunaga NH, Jadeja RN, Rachakonda V, Ahmad D, McLean LP, Cheng K, et al. M1 muscarinic receptors modify oxidative stress response to acetaminophen-induced acute liver injury. Free Radic Biol Med. 2015; 78:66–81.22. Rachakonda V, Jadeja RN, Urrunaga NH, Shah N, Ahmad D, Cheng K, et al. M1 muscarinic receptor deficiency attenuates azoxymethane-induced chronic liver injury in mice. Sci Rep. 2015; 5:14110.23. Steers W, Corcos J, Foote J, Kralidis G. An investigation of dose titration with darifenacin, an M3-selective receptor antagonist. BJU Int. 2005; 95:580–586.24. Kelleher CJ, Cardozo LD, Khullar V, Salvatore S. A mediumterm analysis of the subjective efficacy of treatment for women with detrusor instability and low bladder compliance. Br J Obstet Gynaecol. 1997; 104:988–993.25. Boccuzzi SJ, Le TK, Wogan T. Utilization patterns associated with tolterodine IR vs. oxybutinin in the management of urinary incontinence. Value Health. 2002; 5:274.26. Tarcan T, Akbal C, Sekerci CA, Top T, Simşek F. Intradetrusor injections of onabotulinum toxin-A in children with urinary incontinence due to neurogenic detrusor overactivity refractory to antimuscarinic treatment. Korean J Urol. 2014; 55:281–287.27. Su N, Choi HP, Wang F, Su H, Fei Z, Yang JH, et al. Quantitative proteomic analysis of differentially expressed proteins and downstream signaling pathways in chronic bladder ischemia. J Urol. 2016; 195:515–523.28. Zhang Q, Siroky M, Yang JH, Zhao Z, Azadzoi K. Effects of ischemia and oxidative stress on bladder purinoceptors expression. Urology. 2014; 84:1249.e1–1249.e7.29. Chancellor MB. The overactive bladder progression to underactive bladder hypothesis. Int Urol Nephrol. 2014; 46:Suppl 1. S23–S27.30. Andersson KE. Bladder underactivity. Eur Urol. 2014; 65:399–401.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Corrigendum: Correction of title spelling. Progressive changes in detrusor function and micturition patterns with chronic bladder ischemia
- New therapeutic directions to treat underactive bladder
- Changes of Detrusor Function in Patients with Frontal Lobe Lesion
- The Effect of Botulinum Toxin and Resiniferatoxin on the Detrusor Overactivity Induced by Cyclophosphamide in Rat Bladder
- Urodynamic Findings in an Awake Chemical Cystitis Rat Model Observed by Simultaneous Registrations of Intravesical and Intraabdominal Pressures