Cancer Res Treat.
2018 Oct;50(4):1226-1237. 10.4143/crt.2017.446.
Safety Results and Analysis of Eribulin Efficacy according to Previous Microtubules-Inhibitors Sensitivity in the French Prospective Expanded Access Program for Heavily Pre-treated Metastatic Breast Cancer
- Affiliations
-
- 1Department of Medical Oncology, Institut Paoli-Calmettes, Marseille, France. sabatierr@ipc.unicancer.fr
- 2Aix Marseille University, CNRS, INSERM, Institut Paoli-Calmettes, CRCM, Marseille, France.
- 3Department of Medical Oncology, Institut Curie, Paris, France.
- 4University Hospital Jean Minjoz, INSERM, Besançon, France.
- 5Institut Curie-Hôpital René Huguenin, Saint-Cloud, France.
- 6Institut Claudius-Regaud, IUCT-oncopole, Université Paul-Sabatier, Toulouse, France.
- 7Department of Clinical Research and Innovation, Biostatistics Unit, Institut Paoli-Calmettes, Marseille, France.
- 8Aix Marseille University, INSERM, IRD, SESSTIM, Marseille, France.
- KMID: 2424795
- DOI: http://doi.org/10.4143/crt.2017.446
Abstract
- PURPOSE
Eribulin is approved for advanced breast cancers refractory to anthracyclines and taxanes. Efficacy according to sensitivity to previous therapies has been poorly explored.
MATERIALS AND METHODS
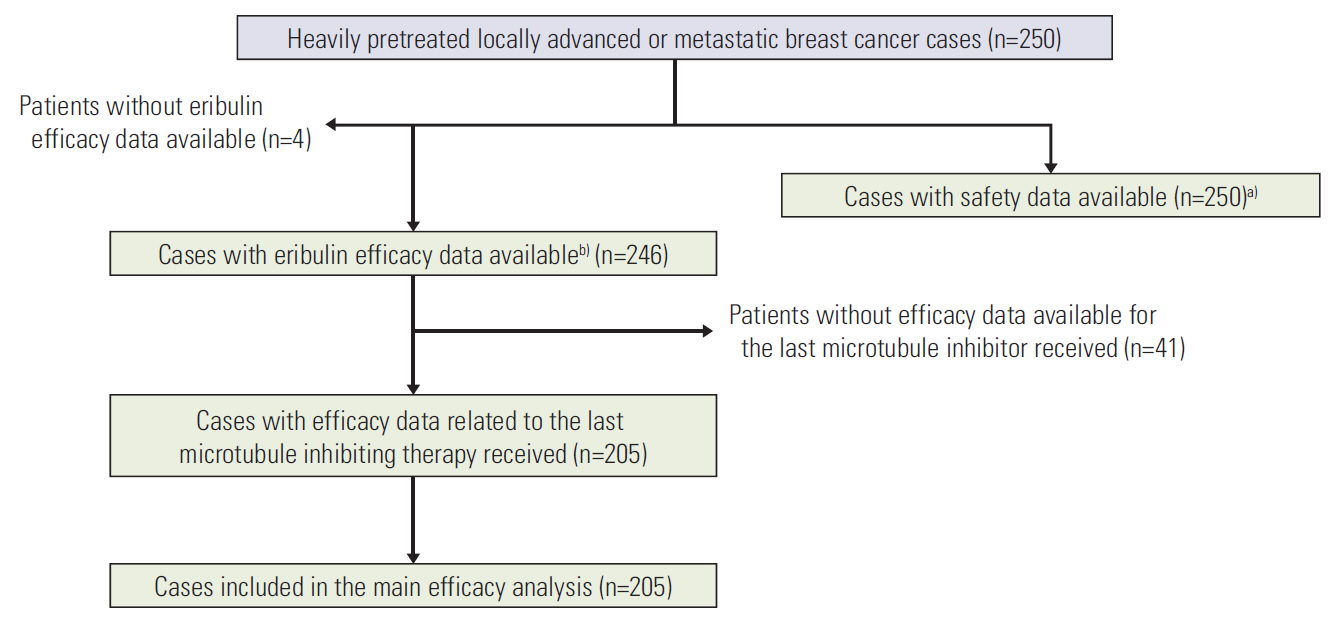
Safety data were collected prospectively and we retrospectively collected efficacy data from the five French centres that participated in the Eribulin E7389-G000-398 expanded access program. Our main objectives were exploration of safety and analysis of eribulin efficacy (progression-free survival [PFS] and overall survival [OS]) according to sensitivity to the last microtubule-inhibiting agent administered.
RESULTS
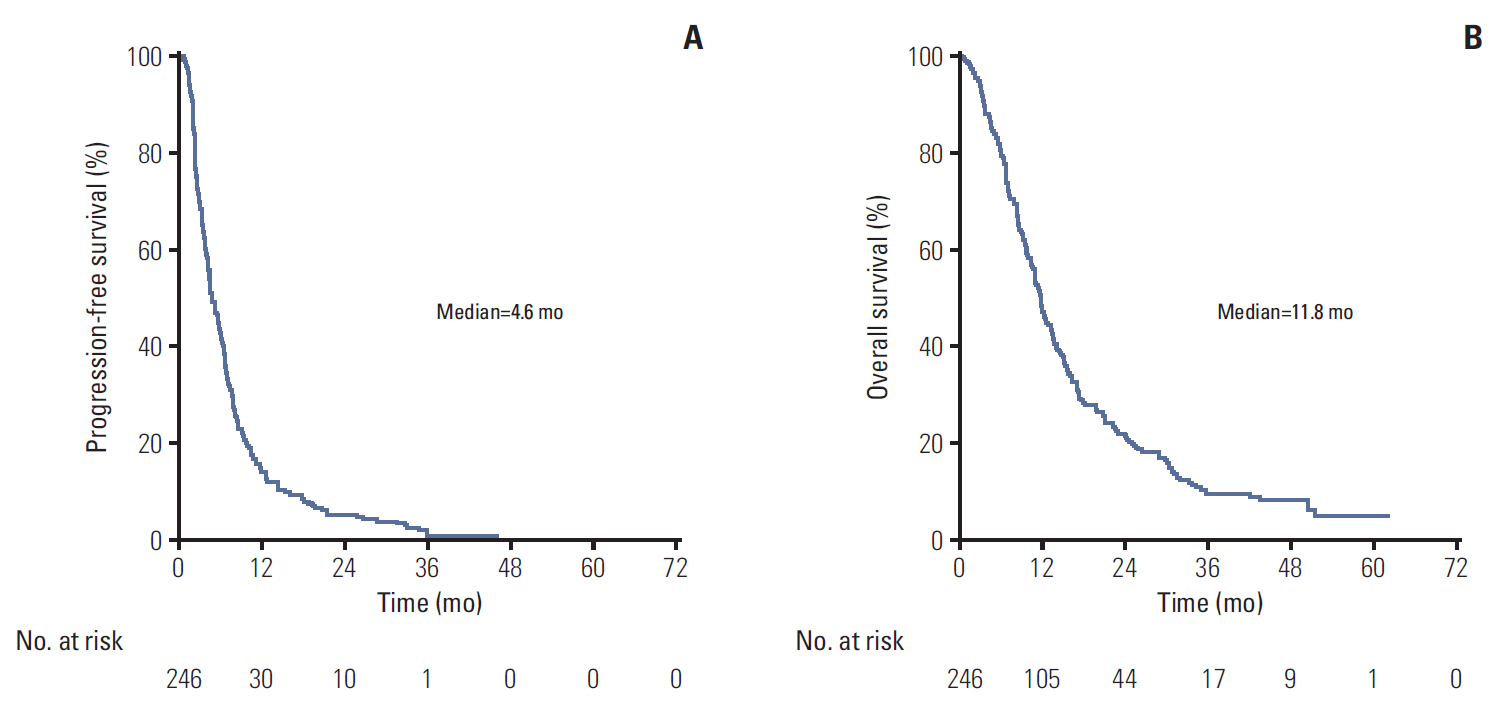
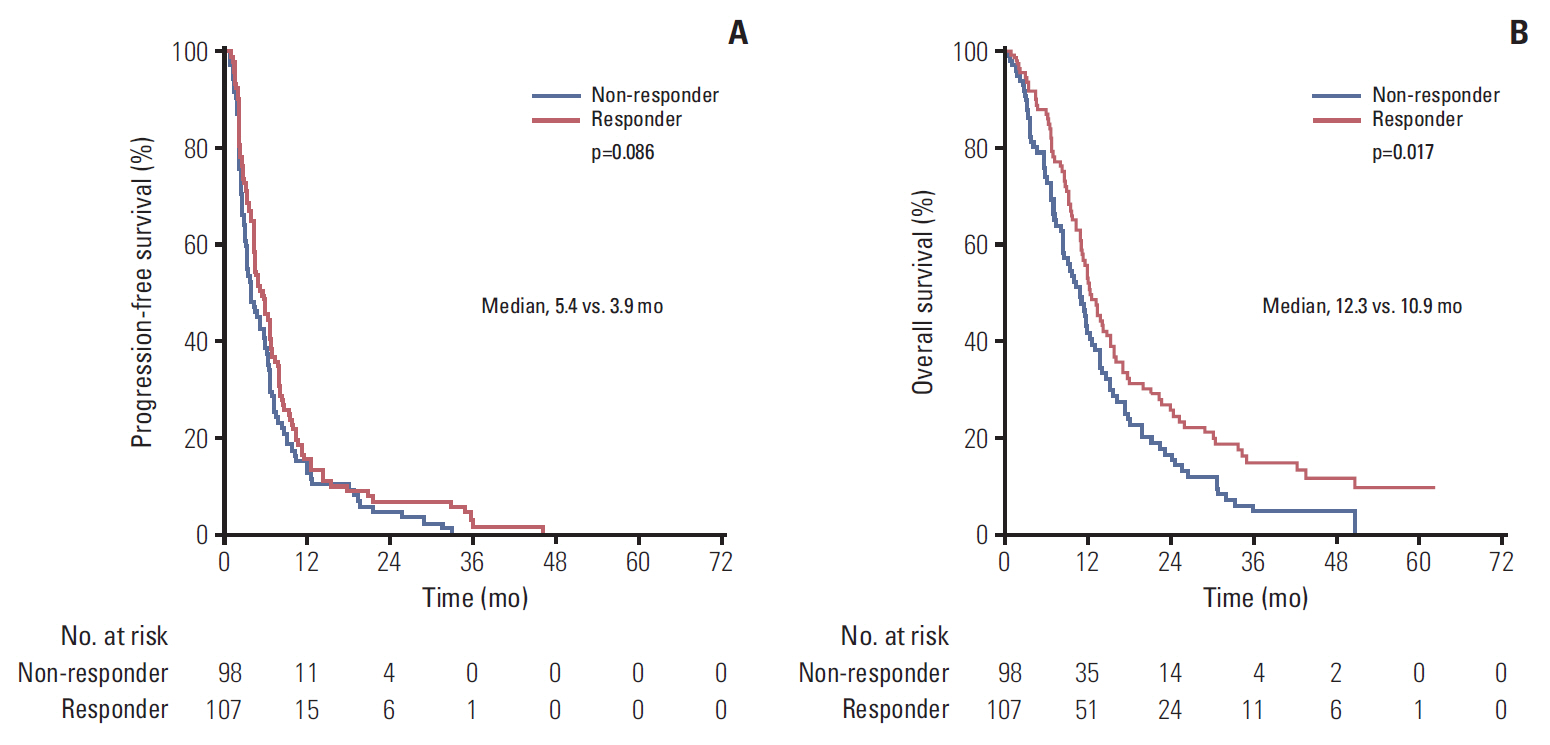
Median eribulin treatment duration was 3.3 months for the 250 patients included in this prospective single-arm study. Two hundreds and thirty-nine patients (95.6%) experienced an adverse event (AE) related to treatment including 129 (51.6%) with grade ≥ 3 AEs. The most frequently observed toxicities were cytopenias (59.6% of included patients), gastro-intestinal disorders (59.2%), and asthenia (56.4%). The most frequent grade 3-4 AE was neutropenia (37.2% with 4.8% febrile neutropenia). Median PFS and OS were 4.6 and 11.8 months, respectively. Patients classified as responders to the last microtubule-inhibiting therapy had a longer OS (hazard ratio [HR], 0.69; 95% confidence interval [CI], 0.51 to 0.94; p=0.017), and tended to display a better PFS (HR, 0.78; 95% CI, 0.58 to 1.04; p=0.086). OS improvement was still significant in multivariate analysis (adjusted HR, 0.53; 95% CI, 0.35 to 0.79; p=0.002).
CONCLUSION
This work based on a prospective study suggests that identification of patients likely to be more sensitive to eribulin could be based on their previous response to microtubules inhibitors.
MeSH Terms
Figure
Reference
-
References
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015; 65:87–108.
Article2. Cardoso F, Costa A, Senkus E, Aapro M, Andre F, Barrios CH, et al. 3rd ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 3). Ann Oncol. 2017; 28:3111.
Article3. Baselga J, Cortes J, Kim SB, Im SA, Hegg R, Im YH, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012; 366:109–19.
Article4. Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006; 355:2733–43.
Article5. Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001; 344:783–92.
Article6. Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012; 367:1783–91.
Article7. Baselga J, Campone M, Piccart M, Burris HA 3rd, Rugo HS, Sahmoud T, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012; 366:520–9.
Article8. Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016; 17:425–39.
Article9. Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015; 16:25–35.
Article10. Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016; 375:1738–48.
Article11. Twelves C, Jove M, Gombos A, Awada A. Cytotoxic chemotherapy: still the mainstay of clinical practice for all subtypes metastatic breast cancer. Crit Rev Oncol Hematol. 2016; 100:74–87.
Article12. Kok VC. Eribulin in the management of advanced breast cancer: implications of current research findings. Breast Cancer (Auckl). 2015; 9:109–15.
Article13. Cortes J, O'Shaughnessy J, Loesch D, Blum JL, Vahdat LT, Petrakova K, et al. Eribulin monotherapy versus treatment of physician's choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet. 2011; 377:914–23.
Article14. Kaufman PA, Awada A, Twelves C, Yelle L, Perez EA, Velikova G, et al. Phase III open-label randomized study of eribulin mesylate versus capecitabine in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2015; 33:594–601.
Article15. Pivot X, Marme F, Koenigsberg R, Guo M, Berrak E, Wolfer A. Pooled analyses of eribulin in metastatic breast cancer patients with at least one prior chemotherapy. Ann Oncol. 2016; 27:1525–31.
Article16. Aogi K, Iwata H, Masuda N, Mukai H, Yoshida M, Rai Y, et al. A phase II study of eribulin in Japanese patients with heavily pretreated metastatic breast cancer. Ann Oncol. 2012; 23:1441–8.
Article17. Inoue K, Saito T, Okubo K, Kimizuka K, Yamada H, Sakurai T, et al. Phase II clinical study of eribulin monotherapy in Japanese patients with metastatic breast cancer who had well-defined taxane resistance. Breast Cancer Res Treat. 2016; 157:295–305.
Article18. Vahdat LT, Pruitt B, Fabian CJ, Rivera RR, Smith DA, Tan-Chiu E, et al. Phase II study of eribulin mesylate, a halichondrin B analog, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2009; 27:2954–61.
Article19. Dell'Ova M, De Maio E, Guiu S, Roca L, Dalenc F, Durigova A, et al. Tumour biology, metastatic sites and taxanes sensitivity as determinants of eribulin mesylate efficacy in breast cancer: results from the ERIBEX retrospective, international, multicenter study. BMC Cancer. 2015; 15:659.20. Aftimos P, Polastro L, Ameye L, Jungels C, Vakili J, Paesmans M, et al. Results of the Belgian expanded access program of eribulin in the treatment of metastatic breast cancer closely mirror those of the pivotal phase III trial. Eur J Cancer. 2016; 60:117–24.
Article21. Jimeno A. Eribulin: rediscovering tubulin as an anticancer target. Clin Cancer Res. 2009; 15:3903–5.
Article22. McBride A, Butler SK. Eribulin mesylate: a novel halichondrin B analogue for the treatment of metastatic breast cancer. Am J Health Syst Pharm. 2012; 69:745–55.
Article23. Twelves C, Cortes J, Vahdat L, Olivo M, He Y, Kaufman PA, et al. Efficacy of eribulin in women with metastatic breast cancer: a pooled analysis of two phase 3 studies. Breast Cancer Res Treat. 2014; 148:553–61.
Article24. Schwartz LH, Litiere S, de Vries E, Ford R, Gwyther S, Mandrekar S, et al. RECIST 1.1-Update and clarification: from the RECIST committee. Eur J Cancer. 2016; 62:132–7.
Article25. Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009; 50 Suppl 1:122S–50S.
Article26. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Prev Med. 2007; 45:247–51.
Article27. Dufresne A, Pivot X, Tournigand C, Facchini T, Altweegg T, Chaigneau L, et al. Impact of chemotherapy beyond the first line in patients with metastatic breast cancer. Breast Cancer Res Treat. 2008; 107:275–9.
Article28. Zelek L, Barthier S, Riofrio M, Fizazi K, Rixe O, Delord JP, et al. Weekly vinorelbine is an effective palliative regimen after failure with anthracyclines and taxanes in metastatic breast carcinoma. Cancer. 2001; 92:2267–72.
Article29. Lopes G, Gluck S, Avancha K, Montero AJ. A cost effectiveness study of eribulin versus standard single-agent cytotoxic chemotherapy for women with previously treated metastatic breast cancer. Breast Cancer Res Treat. 2013; 137:187–93.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Eribulin for Advanced Breast Cancer: A Drug Evaluation
- Eribulin Mesylate Combined with Local Treatment for Brain Metastasis from Breast Cancer: Two Case Reports
- Patient Management with Eribulin in Metastatic Breast Cancer: A Clinical Practice Guide
- Global Expanded Access Program for Pemigatinib in Patients with Previously Treated Locally Advanced or Metastatic Cholangiocarcinoma and Fibroblast Growth Factor Receptor Gene Alterations
- A Real-world Efficacy of Nab-paclitaxel Monotherapy in Metastatic Breast Cancer