Cancer Res Treat.
2024 Jul;56(3):847-855. 10.4143/crt.2023.1197.
Global Expanded Access Program for Pemigatinib in Patients with Previously Treated Locally Advanced or Metastatic Cholangiocarcinoma and Fibroblast Growth Factor Receptor Gene Alterations
- Affiliations
-
- 1Incyte Corporation, Wilmington, DE, USA
- 2Medical University of Vienna, Department of Internal Medicine I, Comprehensive Cancer Center Vienna, Vienna, Austria
- 3Department of Internal Medicine I, Eberhard-Karls University, Tübingen, Germany
- KMID: 2557671
- DOI: http://doi.org/10.4143/crt.2023.1197
Abstract
- Purpose
Pemigatinib is a fibroblast growth factor receptor-2 (FGFR2) inhibitor approved for use in patients with previously treated cholangiocarcinoma (CCA) and FGFR2 fusions or rearrangements. This ongoing global Expanded Access Program (EAP) allows physicians in regions where pemigatinib is not commercially available to request pemigatinib for patients with locally advanced or metastatic CCA who, in the physician’s opinion, could benefit from pemigatinib treatment.
Materials and Methods
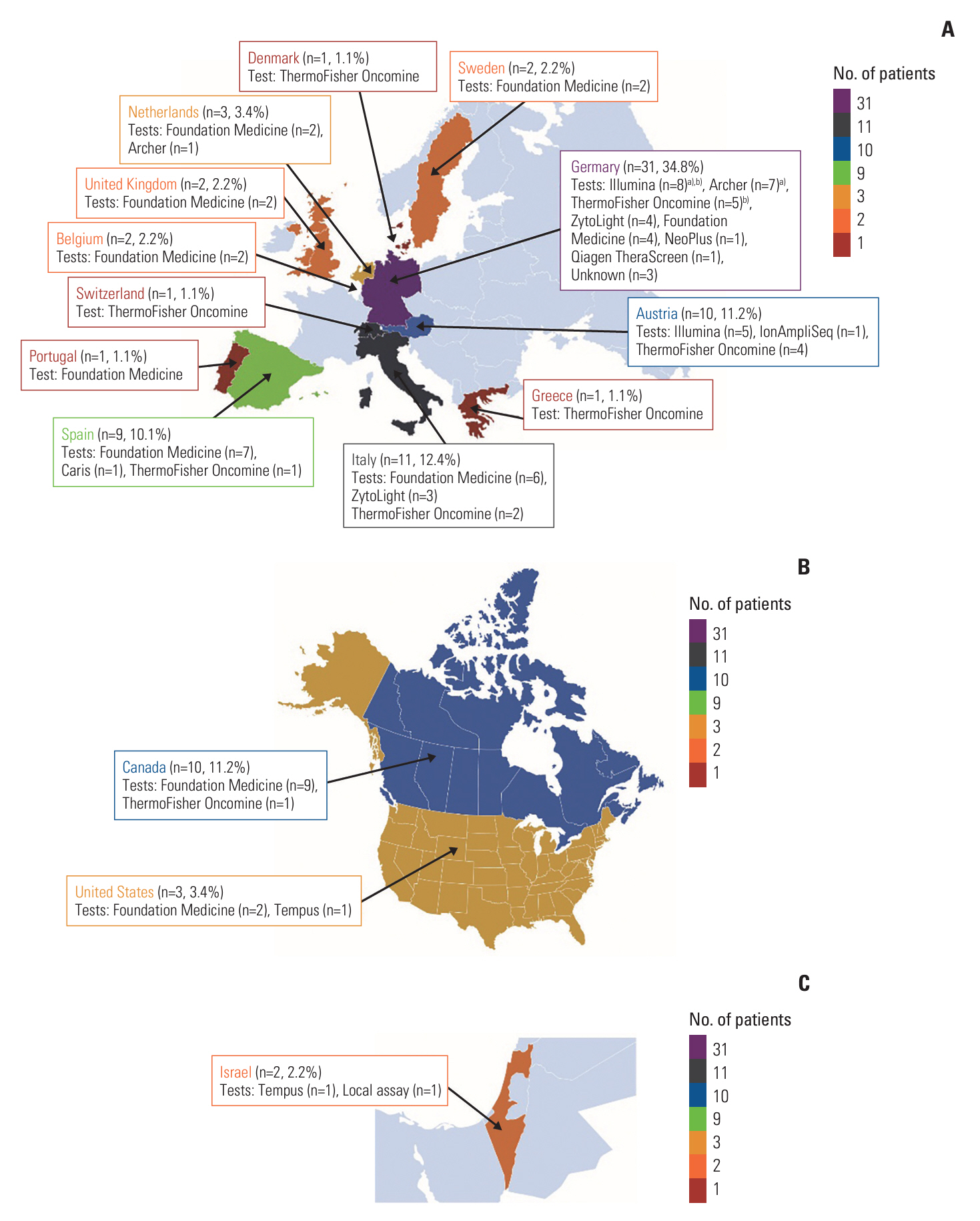
Eighty-nine patients from Europe, North America, and Israel were treated from January 2020 through September 2021.
Results
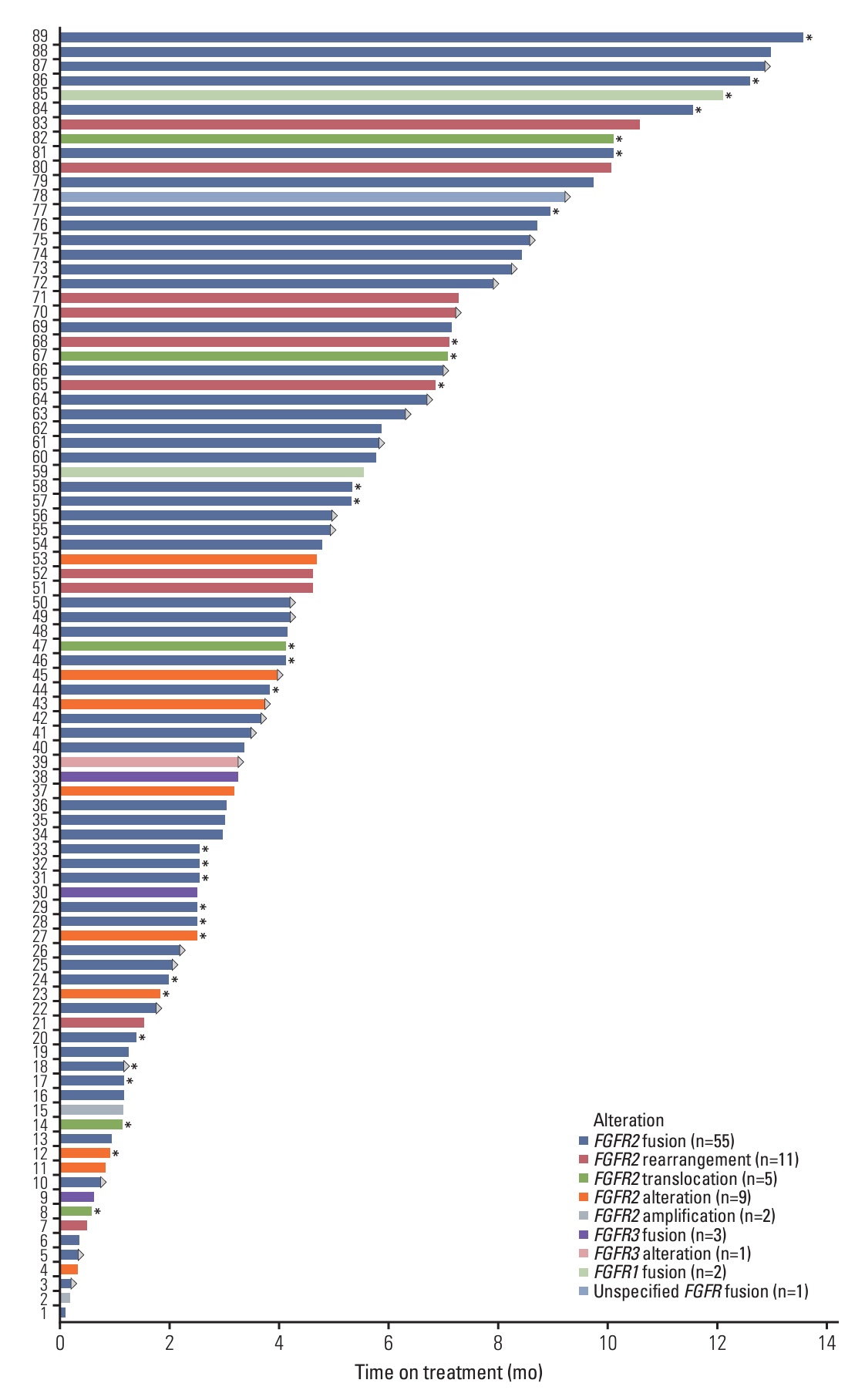
Patients had FGFR gene fusions (68.5%), rearrangements (12.4%), translocations (5.6%), amplifications (2.2%), and other alterations (11.2%). Median duration of treatment in the EAP was 4.0 months (range, 0.1 to 13.6 months). The most frequently reported adverse event (AE) was hyperphosphatemia (22.5%); the most common serious AE was cholangitis (3.4%). Treatment discontinuation was associated with reports of AEs for seven patients (7.9%). AEs associated with pemigatinib were consistent with those observed in clinical trials.
Conclusion
Efficacy was not assessed in this EAP. However, some patients remained on treatment for up to a year, suggesting that they observed a benefit from treatment. Patients with CCA should undergo molecular testing to identify those who could benefit from targeted treatments such as pemigatinib.
Figure
Reference
-
References
1. Louis C, Papoutsoglou P, Coulouarn C. Molecular classification of cholangiocarcinoma. Curr Opin Gastroenterol. 2020; 36:57–62.2. Rumgay H, Ferlay J, de Martel C, Georges D, Ibrahim AS, Zheng R, et al. Global, regional and national burden of primary liver cancer by subtype. Eur J Cancer. 2022; 161:108–18.3. Arai Y, Totoki Y, Hosoda F, Shirota T, Hama N, Nakamura H, et al. Fibroblast growth factor receptor 2 tyrosine kinase fusions define a unique molecular subtype of cholangiocarcinoma. Hepatology. 2014; 59:1427–34.
Article4. Jain A, Borad MJ, Kelley RK, Wang Y, Abdel-Wahab R, MericBernstam F, et al. Cholangiocarcinoma with FGFR genetic aberrations: a unique clinical phenotype. JCO Precis Oncol. 2018; 2:1–12.5. Lowery MA, Ptashkin R, Jordan E, Berger MF, Zehir A, Capanu M, et al. Comprehensive molecular profiling of intrahepatic and extrahepatic cholangiocarcinomas: potential targets for intervention. Clin Cancer Res. 2018; 24:4154–61.
Article6. Javle M, Lee S, Azad NS, Borad MJ, Kate Kelley R, Sivaraman S, et al. Temporal changes in cholangiocarcinoma incidence and mortality in the United States from 2001 to 2017. Oncologist. 2022; 27:874–83.
Article7. Amini N, Ejaz A, Spolverato G, Kim Y, Herman JM, Pawlik TM. Temporal trends in liver-directed therapy of patients with intrahepatic cholangiocarcinoma in the United States: a population-based analysis. J Surg Oncol. 2014; 110:163–70.
Article8. Spolverato G, Kim Y, Alexandrescu S, Marques HP, Lamelas J, Aldrighetti L, et al. Management and outcomes of patients with recurrent intrahepatic cholangiocarcinoma following previous curative-intent surgical resection. Ann Surg Oncol. 2016; 23:235–43.
Article9. Oh DY, Lee KH, Lee DW, Yoon J, Kim TY, Bang JH, et al. Gemcitabine and cisplatin plus durvalumab with or without tremelimumab in chemotherapy-naive patients with advanced biliary tract cancer: an open-label, single-centre, phase 2 study. Lancet Gastroenterol Hepatol. 2022; 7:522–32.
Article10. Vogel A, Bridgewater J, Edeline J, Kelley RK, Klumpen HJ, Malka D, et al. Biliary tract cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023; 34:127–40.
Article11. Liu PC, Koblish H, Wu L, Bowman K, Diamond S, DiMatteo D, et al. INCB054828 (pemigatinib), a potent and selective inhibitor of fibroblast growth factor receptors 1, 2, and 3, displays activity against genetically defined tumor models. PLoS One. 2020; 15:e0231877.
Article12. Abou-Alfa GK, Sahai V, Hollebecque A, Vaccaro G, Melisi D, Al-Rajabi R, et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020; 21:671–84.
Article13. Abou-Alfa GK, Sahai V, Hollebecque A, Vaccaro GM, Melisi D, Al-Rajabi RM, et al. Pemigatinib for previously treated locally advanced/metastatic cholangiocarcinoma (CCA): update of FIGHT-202. J Clin Oncol. 2021; 39(15 Suppl):4086.
Article14. Lowery MA, Goff LW, Keenan BP, Jordan E, Wang R, Bocobo AG, et al. Second-line chemotherapy in advanced biliary cancers: a retrospective, multicenter analysis of outcomes. Cancer. 2019; 125:4426–34.
Article15. Subbiah V, Iannotti NO, Gutierrez M, Smith DC, Feliz L, Lihou CF, et al. FIGHT-101, a first-in-human study of potent and selective FGFR 1-3 inhibitor pemigatinib in pan-cancer patients with FGF/FGFR alterations and advanced malignancies. Ann Oncol. 2022; 33:522–33.16. Ba Y, Deng T, Shi Y, Zhang L, Bai G, Pan Y, et al. A phase 1 study evaluating preliminary safety, pharmacokinetic and pharmacodynamic of pemigatinib in Chinese patients with advanced malignancy. J Clin Oncol. 2021; 39(15 Suppl):e15051.17. Kommalapati A, Tella SH, Borad M, Javle M, Mahipal A. FGFR inhibitors in oncology: insight on the management of toxicities in clinical practice. Cancers (Basel). 2021; 13:2968.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of Fibroblast Growth Factor Receptors Inhibitors in Bladder Cancer
- New systemic treatment options for advanced cholangiocarcinoma
- A case of Pfeiffer syndrome with c833_834GC>TG (Cys278Leu) mutation in the FGFR2 gene
- A case of Apert's Syndrome(Acrocophalosyndactyly) with Fibroblast Growth Factor Receptor 2 Exon IIIa Mutation
- A Case of FGFR2 Exon lllc Mutation in Crouzon Syndrome