J Liver Cancer.
2024 Sep;24(2):155-170. 10.17998/jlc.2024.08.07.
New systemic treatment options for advanced cholangiocarcinoma
- Affiliations
-
- 1Department of Biomedical Sciences, Humanitas University, Milan, Italy
- 2Medical Oncology and Hematology Unit, IRCCS Humanitas Research Hospital, Milan, Italy
- KMID: 2559463
- DOI: http://doi.org/10.17998/jlc.2024.08.07
Abstract
- Cholangiocarcinoma (CCA) is a rare and aggressive cancer, mostly diagnosed at advanced or metastatic stage, at which point systemic treatment represents the only therapeutic option. Chemotherapy has been the backbone of advanced CCA treatment. More recently, immunotherapy has changed the therapeutic landscape, as immune checkpoint inhibitors have yielded the first improvement in survival and currently, the addition of either durvalumab or pembrolizumab to standard of care cisplatin plus gemcitabine represents the new first-line treatment option. However, the use of immunotherapy in subsequent lines has not demonstrated its efficacy and therefore, it is not approved, except for pembrolizumab in the selected microsatellite instability-high population. In addition, advances in comprehensive genomic profiling have led to the identification of targetable genetic alterations, such as isocitrate dehydrogenase 1 (IDH1), fibroblast growth factor receptor 2 (FGFR2), human epidermal growth factor receptor 2 (HER2), proto-oncogene B-Raf (BRAF), neurotrophic tropomyosin receptor kinase (NTRK), rearranged during transfection (RET), Kirsten rat sarcoma virus (KRAS), and mouse double minute 2 homolog (MDM2), thus favoring the development of a precision medicine approach in previously treated patients. Despite these advances, the use of molecularly driven agents is limited to a subgroup of patients. This review aims to provide an overview of the newly approved systemic therapies, the ongoing studies, and future research challenges in advanced CCA management.
Keyword
Figure
Cited by 1 articles
-
A concise review of updated global guidelines for the management of hepatocellular carcinoma: 2017-2024
Hyunjae Shin, Su Jong Yu
J Liver Cancer. 2025;25(1):19-30. doi: 10.17998/jlc.2025.02.03.
Reference
-
References
1. Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020; 17:557–588.
Article2. Ilyas SI, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2018; 15:95–111.3. Khan SA, Tavolari S, Brandi G. Cholangiocarcinoma: epidemiology and risk factors. Liver Int. 2019; 39 Suppl 1:19–31.
Article4. Vithayathil M, Khan SA. Current epidemiology of cholangiocarcinoma in Western countries. J Hepatol. 2022; 77:1690–1698.
Article5. Primrose JN, Fox RP, Palmer DH, Malik HZ, Prasad R, Mirza D, et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019; 20:663–673.
Article6. Vogel A, Bridgewater J, Edeline J, Kelley RK, Klümpen HJ, Malka D, et al. Biliary tract cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023; 34:127–140.
Article7. Benson AB, D’Angelica MI, Abrams T, Abbott DE, Ahmed A, Anaya DA, et al. NCCN guidelines® insights: biliary tract cancers, version 2.2023. J Natl Compr Canc Netw. 2023; 21:694–704.8. Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010; 362:1273–1281.
Article9. Okusaka T, Nakachi K, Fukutomi A, Mizuno N, Ohkawa S, Funakoshi A, et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer. 2010; 103:469–474.
Article10. Valle JW, Furuse J, Jitlal M, Beare S, Mizuno N, Wasan H, et al. Cisplatin and gemcitabine for advanced biliary tract cancer: a meta-analysis of two randomised trials. Ann Oncol. 2014; 25:391–398.
Article11. Morizane C, Okusaka T, Mizusawa J, Katayama H, Ueno M, Ikeda M, et al. Combination gemcitabine plus S-1 versus gemcitabine plus cisplatin for advanced/recurrent biliary tract cancer: the FUGA-BT (JCOG1113) randomized phase III clinical trial. Ann Oncol. 2019; 30:1950–1958.12. Ioka T, Kanai M, Kobayashi S, Sakai D, Eguchi H, Baba H, et al. Randomized phase III study of gemcitabine, cisplatin plus S-1 versus gemcitabine, cisplatin for advanced biliary tract cancer (KHBO1401- MITSUBA). J Hepatobiliary Pancreat Sci. 2023; 30:102–110.13. Phelip JM, Desrame J, Edeline J, Barbier E, Terrebonne E, Michel P, et al. Modified FOLFIRINOX versus CISGEM chemotherapy for patients with advanced biliary tract cancer (PRODIGE 38 AMEBICA): a randomized phase II study. J Clin Oncol. 2022; 40:262–271.
Article14. Shroff RT, Guthrie KA, Scott AJ, Borad MJ, Goff LW, Matin K, et al. SWOG 1815: a phase III randomized trial of gemcitabine, cisplatin, and nab-paclitaxel versus gemcitabine and cisplatin in newly diagnosed, advanced biliary tract cancers. J Clin Oncol. 2023; 41 Suppl 4:LBA490.
Article15. Hawkins MA, Valle JW, Wasan HS, Harrison M, Morement H, Manoharan P, et al. Addition of stereotactic body radiotherapy (SBRT) to systemic chemotherapy in locally advanced cholangiocarcinoma (CC) (ABC-07): results from a randomized phase II trial. J Clin Oncol. 2024; 42 Suppl 16:4006.
Article16. Lamarca A, Palmer DH, Wasan HS, Ross PJ, Ma YT, Arora A, et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): a phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021; 22:690–701.
Article17. Choi IS, Kim KH, Lee JH, Suh KJ, Kim JW, Park JH, et al. A randomised phase II study of oxaliplatin/5-FU (mFOLFOX) versus irinotecan/5- FU (mFOLFIRI) chemotherapy in locally advanced or metastatic biliary tract cancer refractory to first-line gemcitabine/cisplatin chemotherapy. Eur J Cancer. 2021; 154:288–295.18. Yoo C, Kim KP, Jeong JH, Kim I, Kang MJ, Cheon J, et al. Liposomal irinotecan plus fluorouracil and leucovorin versus fluorouracil and leucovorin for metastatic biliary tract cancer after progression on gemcitabine plus cisplatin (NIFTY): a multicentre, open-label, randomised, phase 2b study. Lancet Oncol. 2021; 22:1560–1572.
Article19. Vogel A, Wenzel P, Folprecht G, Schütt P, Wege H, Kretzschmar A, et al. 53MO Nal-IRI and 5-FU/LV compared to 5-FU/LV in patients with cholangio- and gallbladder carcinoma previously treated with gemcitabinebased therapies (NALIRICC - AIO-HEP-0116). Ann Oncol. 2022; 33 Suppl 7:S563–S564.
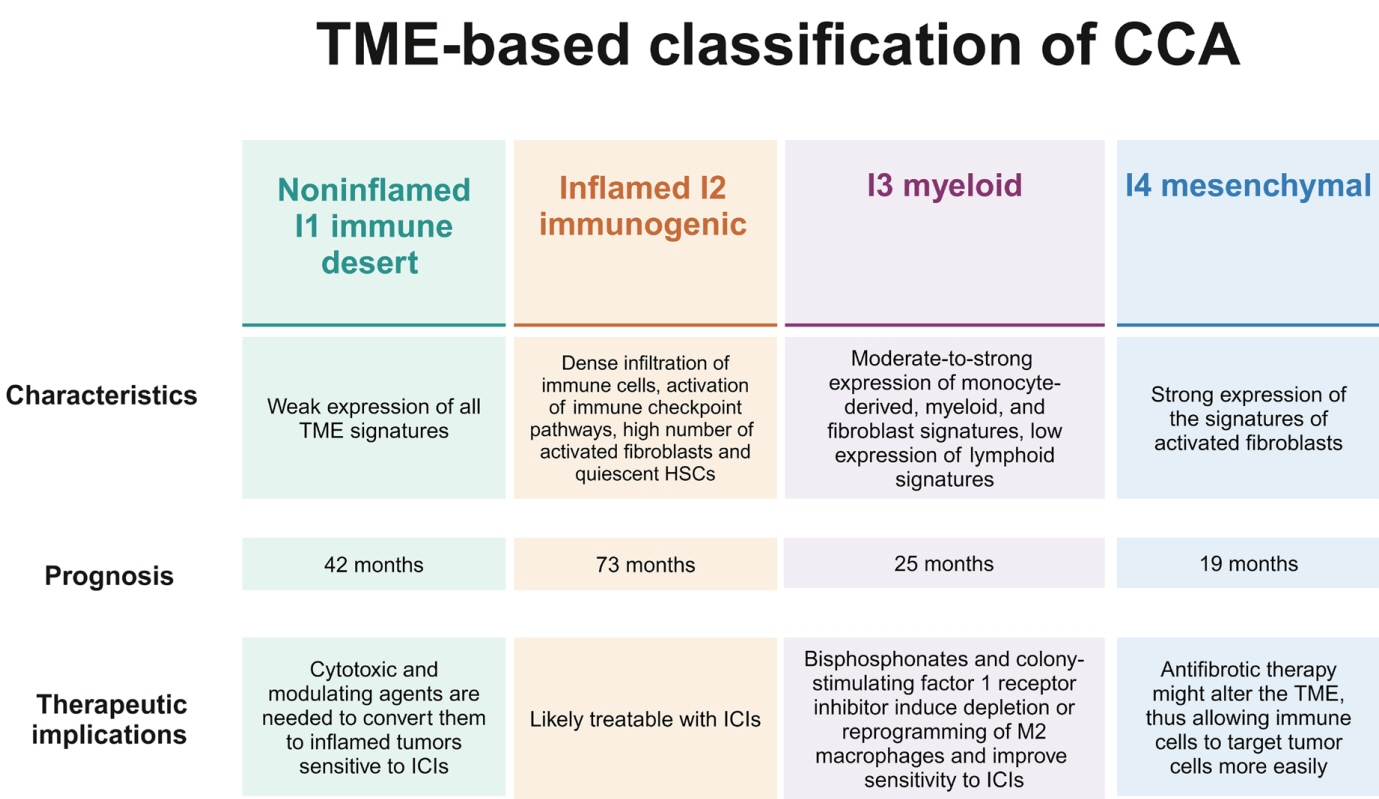
Article20. Job S, Rapoud D, Dos Santos A, Gonzalez P, Desterke C, Pascal G, et al. Identification of four immune subtypes characterized by distinct composition and functions of tumor microenvironment in intrahepatic cholangiocarcinoma. Hepatology. 2020; 72:965–981.
Article21. Bang YJ, Ueno M, Malka D, Chung HC, Nagrial A, Kelley RK, et al. Pembrolizumab (pembro) for advanced biliary adenocarcinoma: results from the KEYNOTE-028 (KN028) and KEYNOTE-158 (KN158) basket studies. J Clin Oncol. 2019; 37 Suppl 15:4079.
Article22. Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord JP, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2020; 38:1–10.23. Maio M, Ascierto PA, Manzyuk L, Motola-Kuba D, Penel N, Cassier PA, et al. Pembrolizumab in microsatellite instability high or mismatch repair deficient cancers: updated analysis from the phase II KEYNOTE-158 study. Ann Oncol. 2022; 33:929–938.
Article24. Kim RD, Chung V, Alese OB, El-Rayes BF, Li D, Al-Toubah TE, et al. A phase 2 multi-institutional study of nivolumab for patients with advanced refractory biliary tract cancer. JAMA Oncol. 2020; 6:888–894.
Article25. Klein O, Kee D, Nagrial A, Markman B, Underhill C, Michael M, et al. Combination immunotherapy with ipilimumab and nivolumab in patients with advanced biliary tract cancers. J Clin Oncol. 2020; 38 Suppl 15:4588.
Article26. Delaye M, Assenat E, Dahan L, Blanc JF, Tougeron D, Metges JP, et al. Durvalumab (D) plus tremelimumab (T) immunotherapy in patients (Pts) with advanced biliary tract carcinoma (BTC) after failure of platinumbased chemotherapy (CTx): interim results of the IMMUNOBIL GERCOR D18-1 PRODIGE-57 study. J Clin Oncol. 2022; 40 Suppl 16:4108.
Article27. Oh DY, Lee KH, Lee DW, Yoon J, Kim TY, Bang JH, et al. Gemcitabine and cisplatin plus durvalumab with or without tremelimumab in chemotherapy-naive patients with advanced biliary tract cancer: an open-label, single-centre, phase 2 study. Lancet Gastroenterol Hepatol. 2022; 7:522–532.28. Oh DY, He AR, Qin S, Chen LT, Okusaka T, Vogel A, et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM Evid. 2022; 1:EVIDoa2200015.
Article29. Oh DY, He AR, Bouattour M, Okusaka T, Qin S, Chen LT, et al. Durvalumab or placebo plus gemcitabine and cisplatin in participants with advanced biliary tract cancer (TOPAZ-1): updated overall survival from a randomised phase 3 study. Lancet Gastroenterol Hepatol. 2024; 9:694–704.30. Burris HA 3rd, Okusaka T, Vogel A, Lee MA, Takahashi H, Breder V, et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer (TOPAZ-1): patient-reported outcomes from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2024; 25:626–635.
Article31. Rimini M, Fornaro L, Lonardi S, Niger M, Lavacchi D, Pressiani T, et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer: an early exploratory analysis of real-world data. Liver Int. 2023; 43:1803–1812.
Article32. Rimini M, Masi G, Lonardi S, Nichetti F, Pressiani T, Lavacchi D, et al. Durvalumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin in biliary tract cancer: a real-world retrospective, multicenter study. Target Oncol. 2024; 19:359–370.33. Olkus A, Tomczak A, Berger AK, Rauber C, Puchas P, Wehling C, et al. Durvalumab plus gemcitabine and cisplatin in patients with advanced biliary tract cancer: an exploratory analysis of real-world data. Target Oncol. 2024; 19:213–221.
Article34. Kelley RK, Ueno M, Yoo C, Finn RS, Furuse J, Ren Z, et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2023; 401:1853–1865.35. Finn RS, Ueno M, Yoo C, Ren Z, Furuse J, Kelley RK, et al. Three-year follow-up data from KEYNOTE-966: pembrolizumab (pembro) plus gemcitabine and cisplatin (gem/cis) compared with gem/cis alone for patients (pts) with advanced biliary tract cancer (BTC). J Clin Oncol. 2024; 42 Suppl 16:4093.
Article36. Hack SP, Zhu AX, Wang Y. Augmenting anticancer immunity through combined targeting of angiogenic and PD-1/PD-L1 pathways: challenges and opportunities. Front Immunol. 2020; 11:598877.
Article37. El-Khoueiry AB, Ren Z, Chon H, Park JO, Kim JW, Pressiani T, et al. IMbrave151: a phase 2, randomized, double-blind, placebo-controlled study of atezolizumab with or without bevacizumab in combination with cisplatin plus gemcitabine in patients with untreated, advanced biliary tract cancer. J Clin Oncol. 2023; 41 Suppl 4:491.38. El-Khoueiry AB, Ren Z, Chon HJ, Park JO, Kim JW, Pressiani T, et al. Atezolizumab plus chemotherapy with or without bevacizumab in advanced biliary tract cancer: results from a randomized proof-of-concept phase II trial (IMbrave151). J Clin Oncol. 2024; 42 Suppl 3:435.
Article39. Song Y, Fu Y, Xie Q, Zhu B, Wang J, Zhang B. Anti-angiogenic agents in combination with immune checkpoint inhibitors: a promising strategy for cancer treatment. Front Immunol. 2020; 11:1956.
Article40. Villanueva L, Lwin Z, Chung HC, Gomez-Roca C, Longo F, Yanez E, et al. Lenvatinib plus pembrolizumab for patients with previously treated biliary tract cancers in the multicohort phase II LEAP-005 study. J Clin Oncol. 2021; 39 Suppl 3:321.
Article41. Zhang Q, Liu X, Wei S, Zhang L, Tian Y, Gao Z, et al. Lenvatinib plus PD-1 inhibitors as first-line treatment in patients with unresectable biliary tract cancer: a single-arm, open-label, phase II study. Front Oncol. 2021; 11:751391.
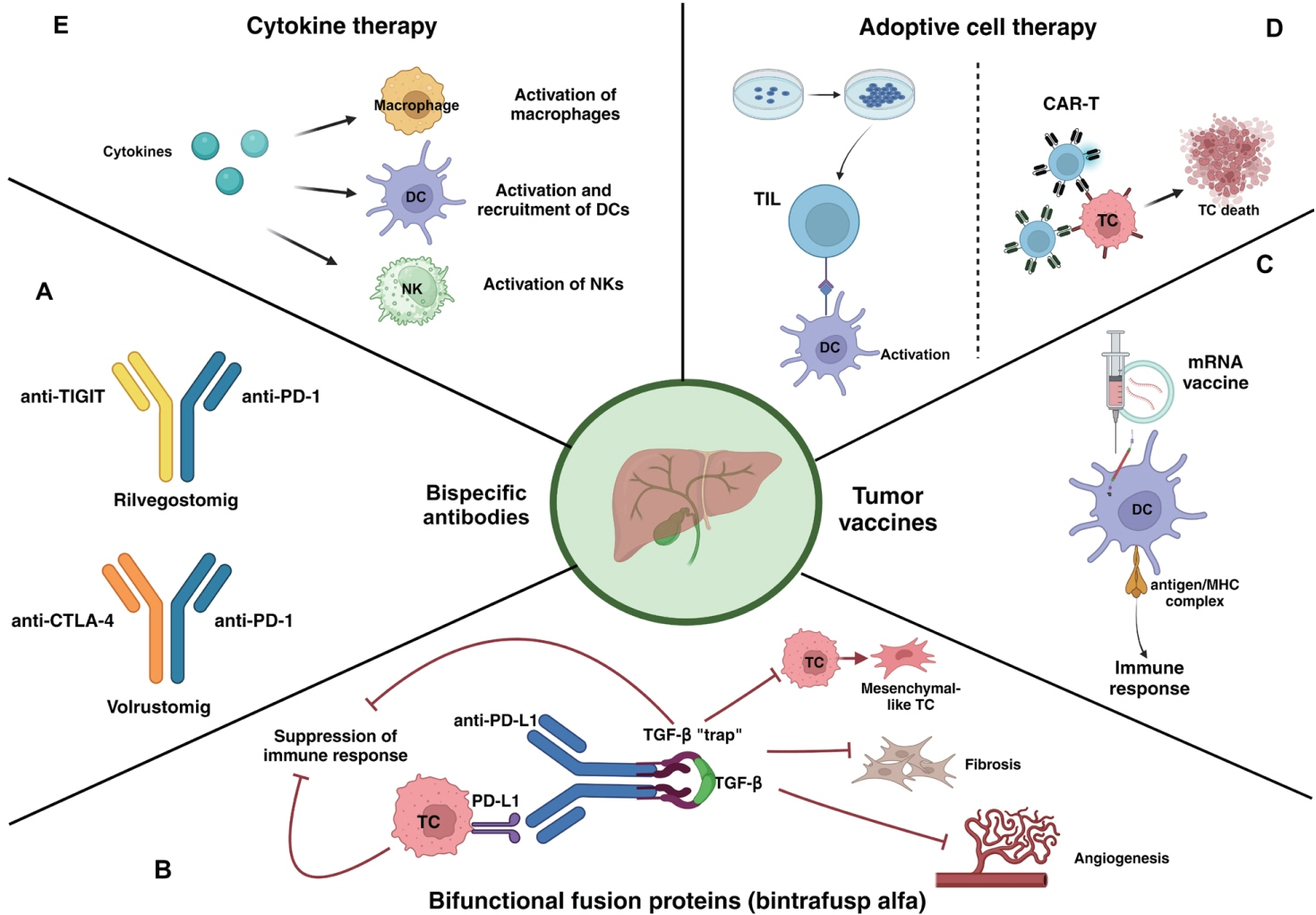
Article42. Rimassa L, Personeni N, Aghemo A, Lleo A. The immune milieu of cholangiocarcinoma: from molecular pathogenesis to precision medicine. J Autoimmun. 2019; 100:17–26.43. Yoo C, Oh DY, Choi HJ, Kudo M, Ueno M, Kondo S, et al. Phase I study of bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in patients with pretreated biliary tract cancer. J Immunother Cancer. 2020; 8:e000564.
Article44. Yoo C, Javle MM, Mata HV, de Braud F, Trojan J, Raoul JL, et al. Phase 2 trial of bintrafusp alfa as second-line therapy for patients with locally advanced/metastatic biliary tract cancers. Hepatology. 2023; 78:758–770.
Article45. Yamamoto K, Ueno T, Kawaoka T, Hazama S, Fukui M, Suehiro Y, et al. MUC1 peptide vaccination in patients with advanced pancreas or biliary tract cancer. Anticancer Res. 2005; 25:3575–3579.46. Kaida M, Morita-Hoshi Y, Soeda A, Wakeda T, Yamaki Y, Kojima Y, et al. Phase 1 trial of Wilms tumor 1 (WT1) peptide vaccine and gemcitabine combination therapy in patients with advanced pancreatic or biliary tract cancer. J Immunother. 2011; 34:92–99.
Article47. Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov. 2018; 17:261–279.48. Huang X, Tang T, Zhang G, Liang T. Identification of tumor antigens and immune subtypes of cholangiocarcinoma for mRNA vaccine development. Mol Cancer. 2021; 20:50.
Article49. Shimizu K, Kotera Y, Aruga A, Takeshita N, Takasaki K, Yamamoto M. Clinical utilization of postoperative dendritic cell vaccine plus activated T-cell transfer in patients with intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Sci. 2012; 19:171–178.
Article50. Guo Y, Feng K, Liu Y, Wu Z, Dai H, Yang Q, et al. Phase I study of chimeric antigen receptor-modified T cells in patients with EGFR-positive advanced biliary tract cancers. Clin Cancer Res. 2018; 24:1277–1286.
Article51. Kelley RK, Bracci PM, Keenan B, Behr S, Ibrahim F, Pollak M, et al. Pembrolizumab (PEM) plus granulocyte macrophage colony stimulating factor (GM-CSF) in advanced biliary cancers (ABC): final outcomes of a phase 2 trial. J Clin Oncol. 2022; 40 Suppl 4:444.
Article52. Lamarca A, Barriuso J, McNamara MG, Valle JW. Molecular targeted therapies: ready for “prime time” in biliary tract cancer. J Hepatol. 2020; 73:170–185.53. Kendre G, Murugesan K, Brummer T, Segatto O, Saborowski A, Vogel A. Charting co-mutation patterns associated with actionable drivers in intrahepatic cholangiocarcinoma. J Hepatol. 2023; 78:614–626.
Article54. Wu MJ, Shi L, Merritt J, Zhu AX, Bardeesy N. Biology of IDH mutant cholangiocarcinoma. Hepatology. 2022; 75:1322–1337.
Article55. Wintheiser G, Zemla T, Shi Q, Tran N, Prasai K, Tella SH, et al. Isocitrate dehydrogenase-mutated cholangiocarcinoma: natural history and clinical outcomes. JCO Precis Oncol. 2022; 6:e2100156.
Article56. Rimini M, Fabregat-Franco C, Persano M, Burgio V, Bergamo F, Niger M, et al. Clinical outcomes after progression on first-line therapies in IDH1 mutated versus wild-type intrahepatic cholangiocarcinoma patients. Target Oncol. 2023; 18:139–145.
Article57. Lowery MA, Burris HA 3rd, Janku F, Shroff RT, Cleary JM, Azad NS, et al. Safety and activity of ivosidenib in patients with IDH1-mutant advanced cholangiocarcinoma: a phase 1 study. Lancet Gastroenterol Hepatol. 2019; 4:711–720.58. Abou-Alfa GK, Macarulla T, Javle MM, Kelley RK, Lubner SJ, Adeva J, et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebocontrolled, phase 3 study. Lancet Oncol. 2020; 21:796–807.
Article59. Zhu AX, Macarulla T, Javle MM, Kelley RK, Lubner SJ, Adeva J, et al. Final overall survival efficacy results of ivosidenib for patients with advanced cholangiocarcinoma with IDH1 mutation: the phase 3 randomized clinical ClarIDHy trial. JAMA Oncol. 2021; 7:1669–1677.
Article60. Valle J, Abou-Alfa G, Kelley R, Lowery M, Shroff R, Bian Y, et al. Quantitative risk-benefit assessment of ivosidenib compared to placebo in patients with IDH1-mutated intrahepatic cholangiocarcinoma: phase 3 ClarIDHy trial. Ann Oncol. 2023; 34 Suppl 1:S162.61. Lapin M, Huang HJ, Chagani S, Javle M, Shroff RT, Pant S, et al. Monitoring of dynamic changes and clonal evolution in circulating tumor DNA from patients with IDH-mutated cholangiocarcinoma treated with isocitrate dehydrogenase inhibitors. JCO Precis Oncol. 2022; 6:e2100197.62. Rimini M, Burgio V, Antonuzzo L, Rimassa L, Oneda E, Soldà C, et al. Updated survival outcomes with ivosidenib in patients with previously treated IDH1-mutated intrahepatic-cholangiocarcinoma: an Italian realworld experience. Ther Adv Med Oncol. 2023; 15:17588359231171574.63. Cleary JM, Rouaisnel B, Daina A, Raghavan S, Roller LA, Huffman BM, et al. Secondary IDH1 resistance mutations and oncogenic IDH2 mutations cause acquired resistance to ivosidenib in cholangiocarcinoma. NPJ Precis Oncol. 2022; 6:61.
Article64. Salati M, Caputo F, Baldessari C, Galassi B, Grossi F, Dominici M, et al. IDH signalling pathway in cholangiocarcinoma: from biological rationale to therapeutic targeting. Cancers (Basel). 2020; 12:3310.
Article65. Vogel A, Segatto O, Stenzinger A, Saborowski A. FGFR2 inhibition in cholangiocarcinoma. Annu Rev Med. 2023; 74:293–306.
Article66. Goyal L, Kongpetch S, Crolley VE, Bridgewater J. Targeting FGFR inhibition in cholangiocarcinoma. Cancer Treat Rev. 2021; 95:102170.
Article67. Abou-Alfa GK, Sahai V, Hollebecque A, Vaccaro G, Melisi D, Al-Rajabi R, et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020; 21:671–684.68. Vogel A, Sahai V, Hollebecque A, Vaccaro GM, Melisi D, Al Rajabi RM, et al. An open-label study of pemigatinib in cholangiocarcinoma: final results from FIGHT-202. ESMO Open. 2024; 9:103488.
Article69. Bekaii-Saab TS, Valle JW, Van Cutsem E, Rimassa L, Furuse J, Ioka T, et al. FIGHT-302: first-line pemigatinib vs gemcitabine plus cisplatin for advanced cholangiocarcinoma with FGFR2 rearrangements. Future Oncol. 2020; 16:2385–2399.
Article70. Parisi A, Delaunay B, Pinterpe G, Hollebecque A, Blanc JF, Bouattour M, et al. Pemigatinib for patients with previously treated, locally advanced or metastatic cholangiocarcinoma harboring FGFR2 fusions or rearrangements: a joint analysis of the French PEMI-BIL and Italian PEMIREAL cohort studies. Eur J Cancer. 2024; 200:113587.
Article71. Goyal L, Meric-Bernstam F, Hollebecque A, Valle JW, Morizane C, Karasic TB, et al. Futibatinib for FGFR2-rearranged intrahepatic cholangiocarcinoma. N Engl J Med. 2023; 388:228–239.
Article72. Hollebecque A, Goyal L, Meric-Bernstam F, Furuse J, Moehler M, Vogel A, et al. Futibatinib in patients with FGFR2-rearranged intrahepatic cholangiocarcinoma: responder analyses of efficacy and safety from the phase 2 FOENIX-CCA2 study. Ann Oncol. 2023; 34 Suppl 1:S163.73. Borad MJ, Bridgewater JA, Morizane C, Shroff RT, Oh DY, Moehler MH, et al. A phase III study of futibatinib (TAS-120) versus gemcitabinecisplatin (gem-cis) chemotherapy as first-line (1L) treatment for patients (pts) with advanced (adv) cholangiocarcinoma (CCA) harboring fibroblast growth factor receptor 2 (FGFR2) gene rearrangements (FOENIXCCA3). J Clin Oncol. 2020; 38 Suppl 4:TPS600.74. Macarulla T, Mizuno T, Brandi G, Li J, Chen MH, Kang JH, et al. FOENIXCCA4: a phase 2 study of futibatinib 20 mg and 16 mg in patients with advanced cholangiocarcinoma (CCA) and fibroblast growth factor receptor 2 (FGFR2) fusions/rearrangements. J Clin Oncol. 2024; 42 Suppl 3:TPS572.75. Javle M, Kelley RK, Roychowdhury S, Weiss KH, Abou-Alfa GK, Macarulla T, et al. A phase II study of infigratinib (BGJ398) in previouslytreated advanced cholangiocarcinoma containing FGFR2 fusions. Hepatobiliary Surg Nutr. 2019; 8 Suppl 1:AB051.76. Javle M, Roychowdhury S, Kelley RK, Sadeghi S, Macarulla T, Weiss KH, et al. Infigratinib (BGJ398) in previously treated patients with advanced or metastatic cholangiocarcinoma with FGFR2 fusions or rearrangements: mature results from a multicentre, open-label, single-arm, phase 2 study. Lancet Gastroenterol Hepatol. 2021; 6:803–815.
Article77. Makawita S, Abou-Alfa GK, Roychowdhury S, Sadeghi S, Borbath I, Goyal L, et al. Infigratinib in patients with advanced cholangiocarcinoma with FGFR2 gene fusions/translocations: the PROOF 301 trial. Future Oncol. 2020; 16:2375–2384.78. Abou-Alfa GK, Borbath I, Roychowdhury S, Goyal L, Lamarca A, Macarulla T, et al. PROOF 301: results of an early discontinued randomized phase 3 trial of the oral FGFR inhibitor infigratinib vs. gemcitabine plus cisplatin in patients with advanced cholangiocarcinoma (CCA) with an FGFR2 gene fusion/rearrangement. Clin Oncol. 2024; 42 Suppl 3:516.79. Mazzaferro V, El-Rayes BF, Droz Dit Busset M, Cotsoglou C, Harris WP, Damjanov N, et al. Derazantinib (ARQ 087) in advanced or inoperable FGFR2 gene fusion-positive intrahepatic cholangiocarcinoma. Br J Cancer. 2019; 120:165–171.
Article80. Borad M, Javle M, Shaib WL, Mody K, Bergamo F, Harris WP, et al. Efficacy of derazantinib in intrahepatic cholangiocarcinoma (iCCA) patients with FGFR2 fusions, mutations or amplifications. Ann Oncol. 2022; 33 Suppl 7:S567–S568.81. Park JO, Feng YH, Chen YY, Su WC, Oh DY, Shen L, et al. Updated results of a phase IIa study to evaluate the clinical efficacy and safety of erdafitinib in Asian advanced cholangiocarcinoma (CCA) patients with FGFR alterations. J Clin Oncol. 2019; 37 Suppl 15:4117.
Article82. Pant S, Schuler M, Iyer G, Witt O, Doi T, Qin S, et al. Erdafitinib in patients with advanced solid tumours with FGFR alterations (RAGNAR): an international, single-arm, phase 2 study. Lancet Oncol. 2023; 24:925–935.83. Borad MJ, Schram AM, Kim RD, Kamath SD, Sahai V, Dotan E, et al. Updated dose escalation results for ReFocus, a first-in-human study of highly selective FGFR2 inhibitor RLY-4008 in cholangiocarcinoma and other solid tumors. J Clin Oncol. 2023; 41 Suppl 16:4009.
Article84. Silverman IM, Hollebecque A, Friboulet L, Owens S, Newton RC, Zhen H, et al. Clinicogenomic analysis of FGFR2-rearranged cholangiocarcinoma identifies correlates of response and mechanisms of resistance to pemigatinib. Cancer Discov. 2021; 11:326–339.
Article85. Javle MM, Mahipal A, Fonkoua LAK, Fountzilas C, Li D, Pelster M, et al. Efficacy and safety results of FGFR1-3 inhibitor, tinengotinib, as monotherapy in patients with advanced, metastatic cholangiocarcinoma: results from phase II clinical trial. J Clin Oncol. 2024; 42 Suppl 3:434.
Article86. Zhang P, Gong J, Niu Z, Cheng Y, Fan J, Peng P, et al. Tinengotinib (TT00420) in combination with atezolizumab in Chinese patients (pts) with biliary tract carcinoma (BTC): preliminary efficacy and safety results from a phase Ib/II study. J Clin Oncol. 2024; 42 Suppl 3:473.
Article87. Galdy S, Lamarca A, McNamara MG, Hubner RA, Cella CA, Fazio N, et al. HER2/HER3 pathway in biliary tract malignancies; systematic review and meta-analysis: a potential therapeutic target? Cancer Metastasis Rev. 2017; 36:141–157.88. Hiraoka N, Nitta H, Ohba A, Yoshida H, Morizane C, Okusaka T, et al. Details of human epidermal growth factor receptor 2 status in 454 cases of biliary tract cancer. Hum Pathol. 2020; 105:9–19.
Article89. Javle M, Borad MJ, Azad NS, Kurzrock R, Abou-Alfa GK, George B, et al. Pertuzumab and trastuzumab for HER2-positive, metastatic biliary tract cancer (MyPathway): a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2021; 22:1290–1300.
Article90. Lee CK, Chon HJ, Cheon J, Lee MA, Im HS, Jang JS, et al. Trastuzumab plus FOLFOX for HER2-positive biliary tract cancer refractory to gemcitabine and cisplatin: a multi-institutional phase 2 trial of the Korean Cancer Study Group (KCSG-HB19-14). Lancet Gastroenterol Hepatol. 2023; 8:56–65.
Article91. Meric-Bernstam F, Beeram M, Hamilton E, Oh DY, Hanna DL, Kang YK, et al. Zanidatamab, a novel bispecific antibody, for the treatment of locally advanced or metastatic HER2-expressing or HER2-amplified cancers: a phase 1, dose-escalation and expansion study. Lancet Oncol. 2022; 23:1558–1570.
Article92. Harding JJ, Fan J, Oh DY, Choi HJ, Kim JW, Chang HM, et al. Zanidatamab for HER2-amplified, unresectable, locally advanced or metastatic biliary tract cancer (HERIZON-BTC-01): a multicentre, single-arm, phase 2b study. Lancet Oncol. 2023; 24:772–782.93. Ohba A, Morizane C, Kawamoto Y, Komatsu Y, Ueno M, Kobayashi S, et al. Trastuzumab deruxtecan (T-DXd; DS-8201) in patients (pts) with HER2-expressing unresectable or recurrent biliary tract cancer (BTC): an investigator-initiated multicenter phase 2 study (HERB trial). J Clin Oncol. 2024; 40 Suppl 16:4006.
Article94. Meric-Bernstam F, Makker V, Oaknin A, Oh DY, Banerjee S, GonzálezMartín A, et al. Efficacy and safety of trastuzumab deruxtecan in patients with HER2-expressing solid tumors: primary results from the DESTINY-PanTumor02 phase II trial. J Clin Oncol. 2024; 42:47–58.
Article95. Nakamura Y, Mizuno N, Sunakawa Y, Canon JL, Galsky MD, Hamilton E, et al. Tucatinib and trastuzumab for previously treated human epidermal growth factor receptor 2-positive metastatic biliary tract cancer (SGNTUC-019): a phase II basket study. J Clin Oncol. 2023; 41:5569–5578.
Article96. Ayasun R, Sahin I. Trastuzumab plus FOLFOX for HER2-positive biliary tract cancer. Lancet Gastroenterol Hepatol. 2023; 8:211.
Article97. Harding JJ, Piha-Paul SA, Shah RH, Murphy JJ, Cleary JM, Shapiro GI, et al. Antitumour activity of neratinib in patients with HER2-mutant advanced biliary tract cancers. Nat Commun. 2023; 14:630.98. Subbiah V, Kreitman RJ, Wainberg ZA, Gazzah A, Lassen U, Stein A, et al. Dabrafenib plus trametinib in BRAFV600E-mutated rare cancers: the phase 2 ROAR trial. Nat Med. 2023; 29:1103–1112.
Article99. Meric-Bernstam F, Rothe M, Mangat PK, Garrett-Mayer E, Gutierrez R, Ahn ER, et al. Cobimetinib plus vemurafenib in patients with solid tumors with BRAF mutations: results from the targeted agent and profiling utilization registry study. JCO Precis Oncol. 2023; 7:e2300385.100. Doebele RC, Drilon A, Paz-Ares L, Siena S, Shaw AT, Farago AF, et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020; 21:271–282.
Article101. Hong DS, DuBois SG, Kummar S, Farago AF, Albert CM, Rohrberg KS, et al. Larotrectinib in patients with TRK fusion-positive solid tumours: a pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 2020; 21:531–540.
Article102. Subbiah V, Cassier PA, Siena S, Garralda E, Paz-Ares L, Garrido P, et al. Pan-cancer efficacy of pralsetinib in patients with RET fusion-positive solid tumors from the phase 1/2 ARROW trial. Nat Med. 2022; 28:1640–1645.103. Subbiah V, Wolf J, Konda B, Kang H, Spira A, Weiss J, et al. Tumouragnostic efficacy and safety of selpercatinib in patients with RET fusion-positive solid tumours other than lung or thyroid tumours (LIBRETTO-001): a phase 1/2, open-label, basket trial. Lancet Oncol. 2022; 23:1261–1273.
Article104. Pant S, Yaeger R, Spira AI, Pelster M, Sabari JK, Hafez N, et al. KRYSTAL-1: activity and safety of adagrasib (MRTX849) in patients with advanced solid tumors harboring a KRASG12C mutation. J Clin Oncol. 2023; 41 Suppl 36:425082.105. Macarulla T, Yamamoto N, Tolcher AW, Hafez N, Lugowska I, Ramlau R, et al. Efficacy and safety of brigimadlin (BI 907828), an MDM2–p53 antagonist, in patients (pts) with advanced biliary tract cancer: data from two phase Ia/Ib dose-escalation/expansion trials. J Clin Oncol. 2024; 42 Suppl 3:487.
Article106. Verlingue L, Malka D, Allorant A, Massard C, Ferté C, Lacroix L, et al. Precision medicine for patients with advanced biliary tract cancers: an effective strategy within the prospective MOSCATO-01 trial. Eur J Cancer. 2017; 87:122–130.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recent Updates in the Imaging Diagnosis of Cholangiocarcinoma
- Treatment of advanced stage cholangiocarcinoma: Systemic therapy may be the starting step for radical surgery
- Intrahepatic Cholangiocarcinoma: Evolving Concepts and Medical Treatment
- Surgery for Perihilar Cholangiocarcinoma
- Current treatment outcome of hilar cholangiocarcinoma