J Clin Neurol.
2018 Oct;14(4):454-463. 10.3988/jcn.2018.14.4.454.
Multimodal Assessment of Neural Substrates in Computerized Cognitive Training: A Preliminary Study
- Affiliations
-
- 1Department of Neurology, Bobath Memorial Hospital, Seongnam, Korea.
- 2Department of Neurology, Hallym University Sacred Heart Hospital, Anyang, Korea.
- 3Department of Neurology, Kangwon National University Hospital, Chuncheon, Korea.
- 4Department of Neurology, Seoul National University College of Medicine, Seoul National University Bundang Hospital, Seongnam, Korea. neuroksy@snu.ac.kr
- 5Department of Neurology, Gangnam-gu Haengbok Convalescence Hospital, Seoul, Korea.
- 6Department of Neurology, Incheon Sarang General Hospital, Incheon, Korea.
- KMID: 2424172
- DOI: http://doi.org/10.3988/jcn.2018.14.4.454
Abstract
- BACKGROUND AND PURPOSE
Several studies have validated the clinical efficacy of computerized cognitive training applications. However, few studies have investigated the neural substrates of these training applications using simultaneous multimodal neuroimaging modalities. We aimed to determine the effectiveness of computerized cognitive training and corresponding neural substrates through a multimodal approach.
METHODS
Ten patients with mild cognitive impairment (MCI), six patients with subjective memory impairment (SMI), and 10 normal controls received custom-developed computerized cognitive training in the memory clinic of a university hospital. All of the participants completed 24 sessions of computerized cognitive training, each lasting 40 minutes and performed twice weekly. They were assessed using neuropsychological tests (both computerized and conventional), electroencephalography, fluorodeoxyglucose positron-emission tomography (FDG-PET), volumetric magnetic resonance imaging (MRI), and diffusion-tensor imaging (DTI) at pre- and posttraining.
RESULTS
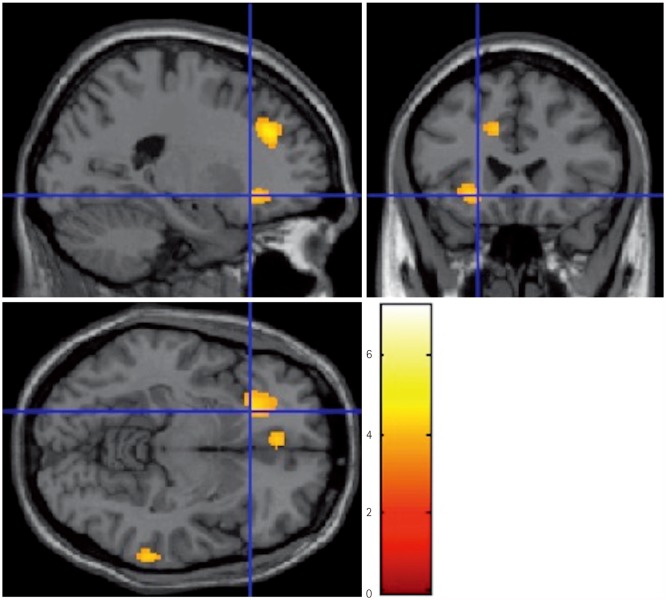
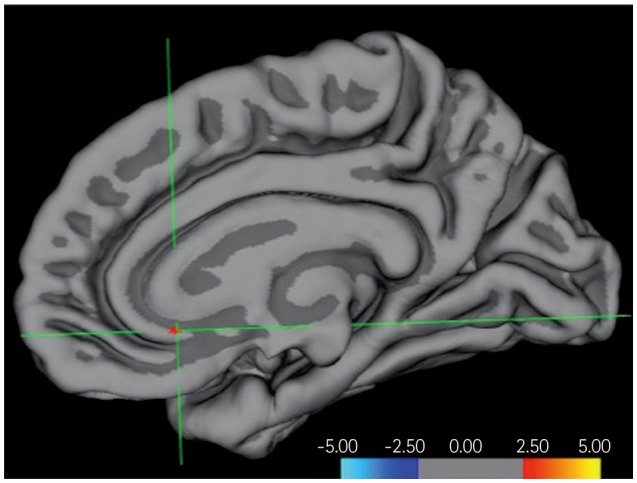
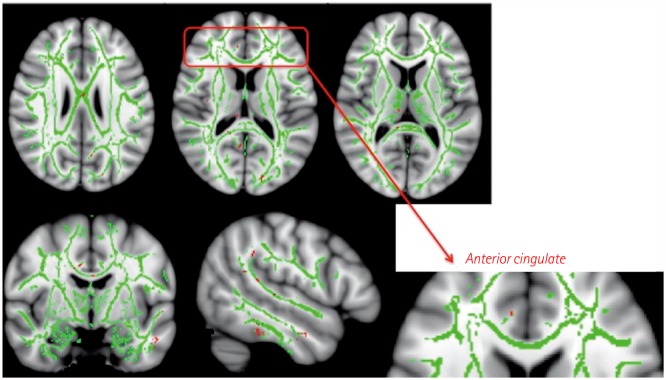
The patients with MCI exhibited significant improvements in the trail-making test-black & white-B, and memory domain of the computerized cognitive assessment. Subjects with normal cognition exhibited significant improvements in scores in the language and attention-/psychomotor-speed domains. There were no significant changes in subjects with SMI. In the pre- and posttraining evaluations of the MCI group, FDG-PET showed focal activation in the left anterior insula and anterior cingulate after training. Volumetric MRI showed a focal increase in the cortical thickness in the rostral anterior cingulate. DTI revealed increased fractional anisotropy in several regions, including the anterior cingulate.
CONCLUSIONS
The anterior cingulate and anterior insula, which are parts of the salience network, may be substrates for the improvements in cognitive function induced by computerized cognitive training.
Keyword
MeSH Terms
Figure
Reference
-
1. Kueider AM, Parisi JM, Gross AL, Rebok GW. Computerized cognitive training with older adults: a systematic review. PLoS One. 2012; 7:e40588. PMID: 22792378.
Article2. Belleville S, Clément F, Mellah S, Gilbert B, Fontaine F, Gauthier S. Training-related brain plasticity in subjects at risk of developing Alzheimer's disease. Brain. 2011; 134:1623–1634. PMID: 21427462.
Article3. Engvig A, Fjell AM, Westlye LT, Skaane NV, Dale AM, Holland D, et al. Effects of cognitive training on gray matter volumes in memory clinic patients with subjective memory impairment. J Alzheimers Dis. 2014; 41:779–791. PMID: 24685630.
Article4. Jeong JH, Na HR, Choi SH, Kim J, Na DL, Seo SW, et al. Group- and home-based cognitive intervention for patients with mild cognitive impairment: a randomized controlled trial. Psychother Psychosom. 2016; 85:198–207. PMID: 27230861.
Article5. Ten Brinke LF, Davis JC, Barha CK, Liu-Ambrose T. Effects of computerized cognitive training on neuroimaging outcomes in older adults: a systematic review. BMC Geriatr. 2017; 17:139. PMID: 28693437.
Article6. Suo C, Singh MF, Gates N, Wen W, Sachdev P, Brodaty H, et al. Therapeutically relevant structural and functional mechanisms triggered by physical and cognitive exercise. Mol Psychiatry. 2016; 21:1645. PMID: 27090304.
Article7. Lampit A, Hallock H, Suo C, Naismith SL, Valenzuela M. Cognitive training-induced short-term functional and long-term structural plastic change is related to gains in global cognition in healthy older adults: a pilot study. Front Aging Neurosci. 2015; 7:14. PMID: 25805989.
Article8. Antonenko D, Külzow N, Cesarz ME, Schindler K, Grittner U, Flöel A. Hippocampal pathway plasticity is associated with the ability to form novel memories in older adults. Front Aging Neurosci. 2016; 8:61. PMID: 27047376.
Article9. Rosen AC, Sugiura L, Kramer JH, Whitfield-Gabrieli S, Gabrieli JD. Cognitive training changes hippocampal function in mild cognitive impairment: a pilot study. J Alzheimers Dis. 2011; 26(Suppl 3):349–357. PMID: 21971474.
Article10. Belleville S, Mellah S, de Boysson C, Demonet JF, Bier B. The pattern and loci of training-induced brain changes in healthy older adults are predicted by the nature of the intervention. PLoS One. 2014; 9:e102710. PMID: 25119464.
Article11. Zatorre RJ, Fields RD, Johansen-Berg H. Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat Neurosci. 2012; 15:528–536. PMID: 22426254.
Article12. Jack CR Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, et al. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013; 12:207–216. PMID: 23332364.
Article13. Polikar R, Tilley C, Hillis B, Clark CM. Multimodal EEG, MRI and PET data fusion for Alzheimer's disease diagnosis. Conf Proc IEEE Eng Med Biol Soc. 2010; 2010:6058–6061. PMID: 21097123.
Article14. Kang Y. [A normative study of the Korean-Mini Mental State Examination (K-MMSE) in the elderly]. Korean J Psychol Gen. 2006; 25:1–12.15. Kang SJ, Choi SH, Lee BH, Kwon JC, Na DL, Han SH, et al. [The reliability and validity of the Korean Instrumental Activities of Daily Living (K-IADL)]. J Korean Neurol Assoc. 2002; 20:8–14.16. Yang DW, Cho BL, Chey JY, Kim SY, Kim BS. [The development and validation of Korean Dementia Screening Questionnaire (KDSQ)]. J Korean Neurol Assoc. 2002; 20:135–141.17. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969; 9:179–186. PMID: 5349366.
Article18. Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, et al. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004; 256:240–246. PMID: 15324367.19. Christensen H. The validity of memory complaints by elderly persons. Int J Geriatr Psychiatry. 1991; 6:307–312.
Article20. Kim HJ, Baek MJ, Kim S. Alternative type of the trail making test in nonnative English-speakers: the trail making test-black & white. PLoS One. 2014; 9:e89078. PMID: 24551221.21. Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002; 15:1–25. PMID: 11747097.
Article22. Boyke J, Driemeyer J, Gaser C, Büchel C, May A. Training-induced brain structure changes in the elderly. J Neurosci. 2008; 28:7031–7035. PMID: 18614670.
Article23. Engvig A, Fjell AM, Westlye LT, Moberget T, Sundseth Ø, Larsen VA, et al. Effects of memory training on cortical thickness in the elderly. Neuroimage. 2010; 52:1667–1676. PMID: 20580844.
Article24. Small GW, Silverman DH, Siddarth P, Ercoli LM, Miller KJ, Lavretsky H, et al. Effects of a 14-day healthy longevity lifestyle program on cognition and brain function. Am J Geriatr Psychiatry. 2006; 14:538–545. PMID: 16731723.
Article25. Hempel A, Giesel FL, Garcia Caraballo NM, Amann M, Meyer H, Wustenberg T, et al. Plasticity of cortical activation related to working memory during training. Am J Psychiatry. 2004; 161:745–747. PMID: 15056524.
Article26. Takeuchi H, Taki Y, Hashizume H, Sassa Y, Nagase T, Nouchi R, et al. Effects of training of processing speed on neural systems. J Neurosci. 2011; 31:12139–12148. PMID: 21865456.
Article27. Engvig A, Fjell AM, Westlye LT, Moberget T, Sundseth Ø, Larsen VA, et al. Memory training impacts short-term changes in aging white matter: a longitudinal diffusion tensor imaging study. Hum Brain Mapp. 2012; 33:2390–2406. PMID: 21823209.
Article28. Roh JH, Park MH, Ko D, Park KW, Lee DH, Han C, et al. Region and frequency specific changes of spectral power in Alzheimer's disease and mild cognitive impairment. Clin Neurophysiol. 2011; 122:2169–2176. PMID: 21715226.
Article29. Summerfield C, Koechlin E. Decision making and prefrontal executive function. In : Gazzaniga MS, Ivry RB, Mangun GR, editors. Cognitive Neuroscience: The Biology of the Mind. 4th ed. Cambridge (MA): The MIT Press;2009. p. 1023–1027.30. Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010; 214:655–667. PMID: 20512370.
Article31. Ros T, Théberge J, Frewen PA, Kluetsch R, Densmore M, Calhoun VD, et al. Mind over chatter: plastic up-regulation of the fMRI salience network directly after EEG neurofeedback. Neuroimage. 2013; 65:324–335. PMID: 23022326.
Article32. Christopher L, Duff-Canning S, Koshimori Y, Segura B, Boileau I, Chen R, et al. Salience network and parahippocampal dopamine dysfunction in memory-impaired Parkinson disease. Ann Neurol. 2015; 77:269–280. PMID: 25448687.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effectiveness of Computerized Cognitive Training Program for Older Adults With Mild Cognitive Impairment: Preliminary Study
- Efficacy of Computerized Cognitive Rehabilitation Training for Inpatients with Schizophrenia : A Pilot Study
- Go and the Brain: Cognitive and Neural Impacts of Training
- A Preliminary Study of Computerized Cognitive Ability Enhancement Program Using Smart-Toy for Children
- Effects of a Computerized Cognitive Training on Cognitive Function, Depression, Self-esteem, and Activities of Daily Living among Older Adults with Mild Cognitive Impairment