Neurointervention.
2018 Sep;13(2):90-99. 10.5469/neuroint.2018.01039.
Characteristic Signs on T2*-Based Imaging and Their Relationship with Results of Reperfusion Therapy for Acute Ischemic Stroke: A Systematic Review and Evidence to Date
- Affiliations
-
- 1Department of Neurosurgery, Kyung Hee University Hospital at Gangdong, School of Medicine, Kyung Hee University, Seoul, Korea.
- 2Department of Radiology, Kyung Hee University Hospital at Gangdong, School of Medicine, Kyung Hee University, Seoul, Korea. md.cwryu@gmail.com
- 3Department of Radiology, Kyung Hee University Hospital, School of Medicine, Kyung Hee University, Seoul, Korea.
- KMID: 2424057
- DOI: http://doi.org/10.5469/neuroint.2018.01039
Abstract
- PURPOSE
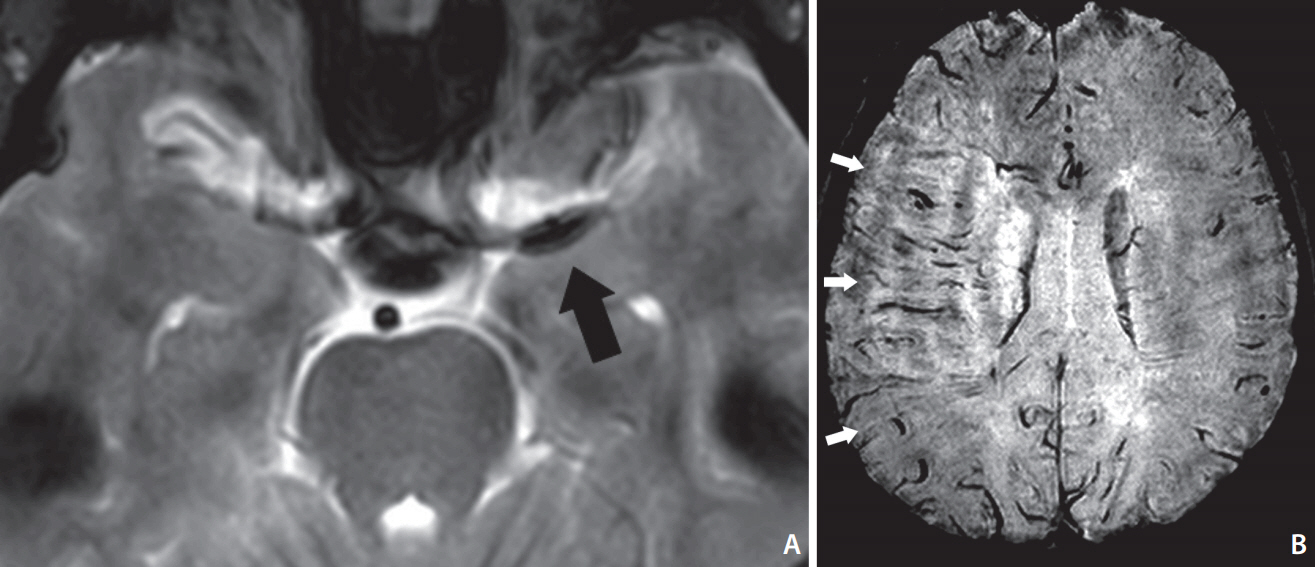
Characteristic signs - the susceptibility vessel sign (SVS) and the prominent hypointense vessel sign (PHVS) - on T2*-based magnetic resonance imaging (T2*MRI) can be seen for acute ischemic stroke with large artery occlusion. In this study, we investigated the evidence to support our hypothesis that these findings may help to predict outcomes after reperfusion therapy.
MATERIALS AND METHODS
We searched for papers describing SVS and PHVS in patients treated with reperfusion therapy for acute ischemic stroke, and their functional/radiologic outcomes were systematically reviewed.
RESULTS
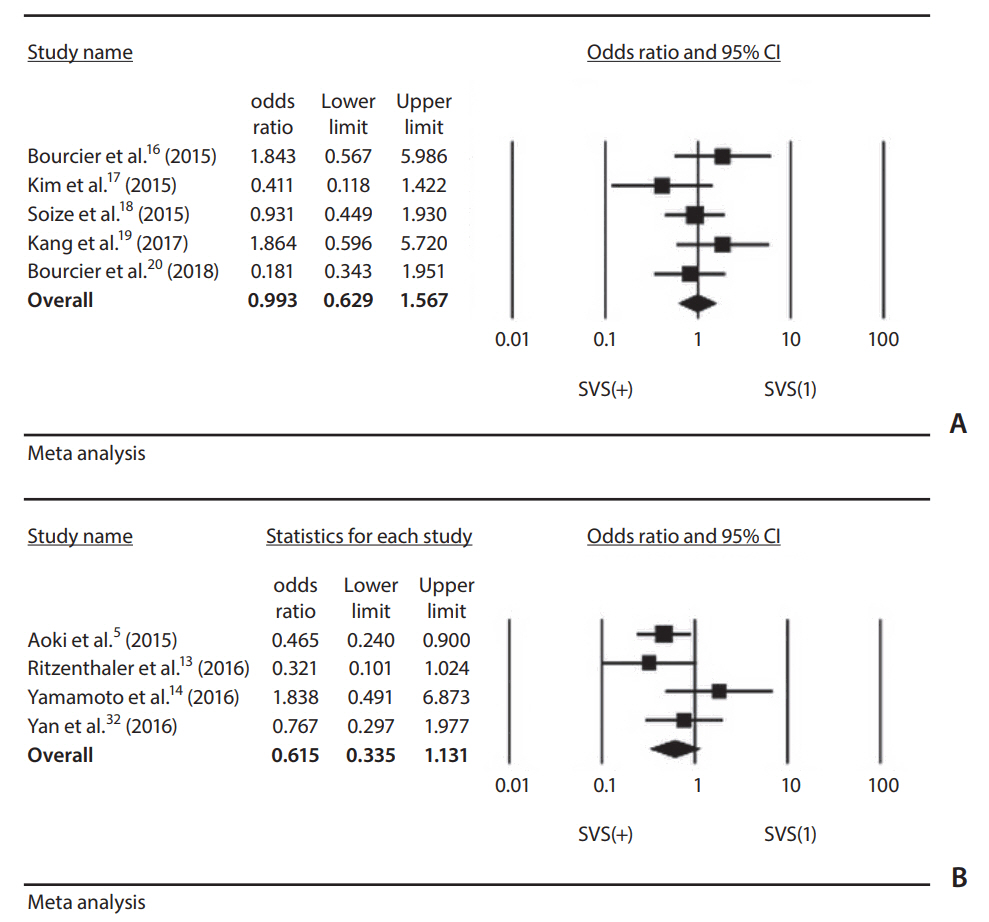
Nine studies on the SVS and six studies on the PHVS were included. The pooled odds ratio (OR) of recanalization after intravenous thrombolysis or mechanical thrombectomy was not significantly different with the presence of SVS (OR, 0.615; 95% confidence interval [CI], 0.335-1.131 and OR, 0.993; 95% CI, 0.629-1.567). The OR of favorable functional outcome after reperfusion therapy in terms of the presence of PHVS varied (0.083 to 1.831) by study.
CONCLUSION
Our meta-analysis of the published data showed that a SVS was not a predictive factor for recanalization after reperfusion therapy for acute ischemic stroke. Currently, the data available on T2*MRI are too limited to warrant reperfusion therapy in routine practice. More data are needed from studies with randomized treatment allocation to determine the role of T2*MRI.
Keyword
Figure
Cited by 1 articles
-
Imaging in Acute Anterior Circulation Ischemic Stroke: Current and Future
Hyun Jeong Kim, Hong Gee Roh
Neurointervention. 2022;17(1):2-17. doi: 10.5469/neuroint.2021.00465.
Reference
-
1. Chavhan GB, Babyn PS, Thomas B, Shroff MM, Haacke EM. Principles, techniques, and applications of T2*-based MR imaging and its special applications. Radiographics. 2009; 29:1433–1449.
Article2. Tsai FY, Kao HW, Tsui YK, Hasso AN, Greensite F. Susceptibility weighted imaging and cerebrovascular disorders. Neuroradiol J. 2011; 24:121–127.
Article3. Kimura K, Iguchi Y, Shibazaki K, Watanabe M, Iwanaga T, Aoki J. M1 susceptibility vessel sign on T2* as a strong predictor for no early recanalization after IV-t-PA in acute ischemic stroke. Stroke. 2009; 40:3130–3132.
Article4. Yan S, Hu H, Shi Z, Zhang X, Zhang S, Liebeskind DS, et al. Morphology of susceptibility vessel sign predicts middle cerebral artery recanalization after intravenous thrombolysis. Stroke. 2014; 45:2795–2797.
Article5. Aoki J, Kimura K, Shibazaki K, Saji N, Uemura J, Sakamoto Y, et al. The susceptibility vessel sign at the proximal M1: a strong predictor for poor outcome after intravenous thrombolysis. J Neurol Sci. 2015; 348:195–200.
Article6. Kao HW, Tsai FY, Hasso AN. Predicting stroke evolution: comparison of susceptibility-weighted MR imaging with MR perfusion. Eur Radiol. 2012; 22:1397–1403.
Article7. Lee JM, Vo KD, An H, Celik A, Lee Y, Hsu CY, et al. Magnetic resonance cerebral metabolic rate of oxygen utilization in hyperacute stroke patients. Ann Neurol. 2003; 53:227–232.
Article8. Charidimou A, Turc G, Oppenheim C, Yan S, Scheitz JF, Erdur H, et al. Microbleeds, cerebral hemorrhage, and functional outcome after stroke thrombolysis: individual patient data meta-analysis. Stroke. 2017; 48:2084–2090.
Article9. Charidimou A, Shoamanesh A; International Meta-Microbleeds Initiative. Clinical relevance of microbleeds in acute stroke thrombolysis: comprehensive meta-analysis. Neurology. 2016; 87:1534–1541.10. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000; 283:2008–2012.11. Zaidat OO, Yoo AJ, Khatri P, Tomsick TA, von Kummer R, Saver JL, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke. 2013; 44:2650–2663.12. Mori E, Minematsu K, Nakagawara J, Yamaguchi T, Sasaki M, Hirano T, et al. Effects of 0.6 mg/kg intravenous alteplase on vascular and clinical outcomes in middle cerebral artery occlusion: Japan Alteplase Clinical Trial II (J-ACT II). Stroke. 2010; 41:461–465.13. Ritzenthaler T, Lacalm A, Cho TH, Maucort-Boulch D, Klaerke Mikkelsen I, Ribe L, et al. Sequential MR assessment of the susceptibility vessel sign and arterial occlusion in acute stroke. J Neuroimaging. 2016; 26:355–359.
Article14. Yamamoto N, Satomi J, Harada M, Izumi Y, Nagahiro S, Kaji R. Is the susceptibility vessel sign on 3-tesla magnetic resonance T2*-weighted imaging a useful tool to predict recanalization in intravenous tissue plasminogen activator? Clin Neuroradiol. 2016; 26:317–323.
Article15. Yan S, Liu K, Tong L, Yu Y, Zhang S, Lou M. Different risk factors for poor outcome between patients with positive and negative susceptibility vessel sign. J Neurointerv Surg. 2016; 8:1001–1005.
Article16. Bourcier R, Volpi S, Guyomarch B, Daumas-Duport B, Lintia-Gaultier A, Papagiannaki C, et al. Susceptibility vessel sign on MRI predicts favorable clinical outcome in patients with anterior circulation acute stroke treated with mechanical thrombectomy. AJNR Am J Neuroradiol. 2015; 36:2346–2353.
Article17. Kim SK, Yoon W, Heo TW, Park MS, Kang HK. Negative susceptibility vessel sign and underlying intracranial atherosclerotic stenosis in acute middle cerebral artery occlusion. AJNR Am J Neuroradiol. 2015; 36:1266–1271.
Article18. Soize S, Batista AL, Rodriguez Regent C, Trystram D, Tisserand M, Turc G, et al. Susceptibility vessel sign on T2* magnetic resonance imaging and recanalization results of mechanical thrombectomy with stent retrievers: a multicentre cohort study. Eur J Neurol. 2015; 22:967–972.
Article19. Kang DW, Jeong HG, Kim DY, Yang W, Lee SH. Prediction of stroke subtype and recanalization using susceptibility vessel sign on susceptibility-weighted magnetic resonance imaging. Stroke. 2017; 48:1554–1559.
Article20. Bourcier R, Mazighi M, Labreuche J, Fahed R, Blanc R, Gory B, et al. Susceptibility vessel sign in the ASTER trial: higher recanalization rate and more favourable clinical outcome after first line stent retriever compared to contact aspiration. J Stroke. 2018; 20:268–276.
Article21. Baik SK, Choi W, Oh SJ, Park KP, Park MG, Yang TI, et al. Change in cortical vessel signs on susceptibility-weighted images after full recanalization in hyperacute ischemic stroke. Cerebrovasc Dis. 2012; 34:206–212.
Article22. Lou M, Chen Z, Wan J, Hu H, Cai X, Shi Z, et al. Susceptibility-diffusion mismatch predicts thrombolytic outcomes: a retrospective cohort study. AJNR Am J Neuroradiol. 2014; 35:2061–2067.
Article23. Terasawa Y, Yamamoto N, Morigaki R, Fujita K, Izumi Y, Satomi J, et al. Brush sign on 3-T T2*-weighted MRI as a potential predictor of hemorrhagic transformation after tissue plasminogen activator therapy. Stroke. 2014; 45:274–276.
Article24. Zhang X, Zhang S, Chen Q, Ding W, Campbell BCV, Lou M. Ipsilateral prominent thalamostriate vein on susceptibility-weighted imaging predicts poor outcome after intravenous thrombolysis in acute ischemic stroke. AJNR Am J Neuroradiol. 2017; 38:875–881.
Article25. Zhao G, Sun L, Wang Z, Wang L, Cheng Z, Lei H, et al. Evaluation of the role of susceptibility-weighted imaging in thrombolytic therapy for acute ischemic stroke. J Clin Neurosci. 2017; 40:175–179.
Article26. Wang Y, Shi T, Chen B, Lin G, Xu Y, Geng Y. Prominent hypointense vessel sign on susceptibility-weighted imaging is associated with clinical outcome in acute ischaemic stroke. Eur Neurol. 2018; 79:231–239.
Article27. Kim SK, Yoon W, Kim TS, Kim HS, Heo TW, Park MS. Histologic analysis of retrieved clots in acute ischemic stroke: correlation with stroke etiology and gradient-echo MRI. AJNR Am J Neuroradiol. 2015; 36:1756–1762.
Article28. Jang IK, Gold HK, Ziskind AA, Fallon JT, Holt RE, Leinbach RC, et al. Differential sensitivity of erythrocyte-rich and platelet-rich arterial thrombi to lysis with recombinant tissue-type plasminogen activator. A possible explanation for resistance to coronary thrombolysis. Circulation. 1989; 79:920–928.
Article29. Sporns PB, Hanning U, Schwindt W, Velasco A, Minnerup J, Zoubi T, et al. Ischemic stroke: what does the histological composition tell us about the origin of the thrombus? Stroke. 2017; 48:2206–2210.30. Hashimoto T, Hayakawa M, Funatsu N, Yamagami H, Satow T, Takahashi JC, et al. Histopathologic analysis of retrieved thrombi associated with successful reperfusion after acute stroke thrombectomy. Stroke. 2016; 47:3035–3037.
Article31. Maekawa K, Shibata M, Nakajima H, Mizutani A, Kitano Y, Seguchi M, et al. Erythrocyte-rich thrombus is associated with reduced number of maneuvers and procedure time in patients with acute ischemic stroke undergoing mechanical thrombectomy. Cerebrovasc Dis Extra. 2018; 8:39–49.
Article32. Yan S, Lou M. Abstract W P43: extent of “blooming effect” predicts middle cerebral artery recanalization after intravenous thrombolysis. Stroke. 2015; 46(Suppl_1):AWP43.
Article33. Huang P, Chen CH, Lin WC, Lin RT, Khor GT, Liu CK. Clinical applications of susceptibility weighted imaging in patients with major stroke. J Neurol. 2012; 259:1426–1432.
Article34. Kesavadas C, Thomas B, Pendharakar H, Sylaja PN. Susceptibility weighted imaging: does it give information similar to perfusion weighted imaging in acute stroke? J Neurol. 2011; 258:932–934.
Article35. Chen CY, Chen CI, Tsai FY, Tsai PH, Chan WP. Prominent vessel sign on susceptibility-weighted imaging in acute stroke: prediction of infarct growth and clinical outcome. PLoS One. 2015; 10:e0131118.
Article36. Sun W, Liu W, Zhang Z, Xiao L, Duan Z, Liu D, et al. Asymmetrical cortical vessel sign on susceptibility-weighted imaging: a novel imaging marker for early neurological deterioration and unfavorable prognosis. Eur J Neurol. 2014; 21:1411–1418.
Article37. Dejobert M, Cazals X, Annan M, Debiais S, Lauvin MA, Cottier JP. Susceptibility-diffusion mismatch in hyperacute stroke: correlation with perfusion-diffusion mismatch and clinical outcome. J Stroke Cerebrovasc Dis. 2016; 25:1760–1766.
Article38. Agarwal A, Vijay K, Thamburaj K, Kanekar S, Kalapos P. Sensitivity of 3D gradient recalled echo susceptibility-weighted imaging technique compared to computed tomography angiography for detection of middle cerebral artery thrombus in acute stroke. Neurol Int. 2014; 6:5521.
Article39. Naggara O, Raymond J, Domingo Ayllon M, Al-Shareef F, Touzé E, Chenoufi M, et al. T2* “susceptibility vessel sign” demonstrates clot location and length in acute ischemic stroke. PLoS One. 2013; 8:e76727.
Article40. Rovira A, Orellana P, Alvarez-Sabín J, Arenillas JF, Aymerich X, Grivé E, et al. Hyperacute ischemic stroke: middle cerebral artery susceptibility sign at echo-planar gradient-echo MR imaging. Radiology. 2004; 232:466–473.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Reperfusion therapy in acute ischemic stroke
- Diagnosis and Treatment of Acute Ischemic Stroke Guided by Stroke MRI
- Fast MRI in Acute Ischemic Stroke: Applications of MRI Acceleration Techniques for MR-Based Comprehensive Stroke Imaging
- Imaging-Based Management of Acute Ischemic Stroke Patients: Current Neuroradiological Perspectives
- A Correlation Study of Parameters from MRI and PET in Acute Stroke