Neurointervention.
2018 Sep;13(2):73-83. 10.5469/neuroint.2018.01011.
New Pathophysiological Considerations on Cerebral Aneurysms
- Affiliations
-
- 1Department of Neurology, Seoul National University Hospital, Seoul, Korea. jungkh@gmail.com
- KMID: 2424055
- DOI: http://doi.org/10.5469/neuroint.2018.01011
Abstract
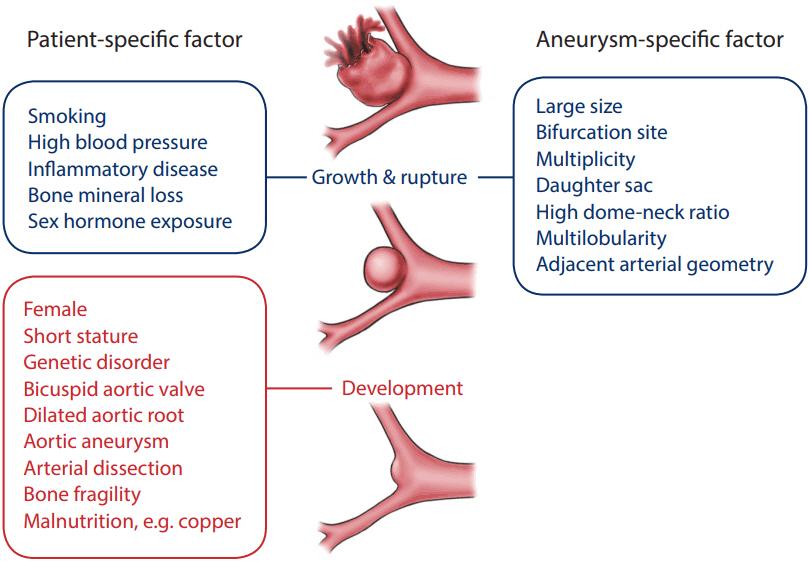
- Cerebral aneurysm is a common cerebrovascular disease that is sometimes complicated by rupture or an enlarged mass. We are now aggressively evaluating and managing unruptured cerebral aneurysms based on a significant concern for the high morbidity and mortality related to its associated complications. However, the actual rupture rate is very low and the diagnostic and treatment modalities are expensive and invasive, which may lead to unnecessary costs and potential medical complications. This disproportionate situation is related to a poor understanding of the natural course and pathophysiology of cerebral aneurysms. In consideration of the concept that not all cerebral aneurysms must be removed, we need to examine their course and progression more accurately. Cerebral aneurysms may follow a variety of pathophysiological scenarios over their lifetime, from formation to growth and rupture. The disease course and the final outcome can differ depending on the timing and intensity of the pathological signals acting on the cerebral vessel wall. We should delineate a method of predicting the stability and risk of rupture of the lesion based on a comprehensive knowledge of the vessel wall integrity. This review deals with the basic knowledge and advanced concepts underlying the pathophysiology of cerebral aneurysms.
Keyword
Figure
Cited by 1 articles
-
How Cerebral Vessel Tortuosity Affects Development and Recurrence of Aneurysm: Outer Curvature versus Bifurcation Type
Hyung Jun Kim, Ha-Na Song, Ji-Eun Lee, Yoon-Chul Kim, In-Young Baek, Ye-Sel Kim, Jong-Won Chung, Tae Keun Jee, Je Young Yeon, Oh Young Bang, Gyeong-Moon Kim, Keon-Ha Kim, Jong-Soo Kim, Seung-Chyul Hong, Woo-Keun Seo, Pyeong Jeon
J Stroke. 2021;23(2):213-222. doi: 10.5853/jos.2020.04399.
Reference
-
1. Vlak MH, Algra A, Brandenburg R, Rinkel GJ. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta-analysis. Lancet Neurol. 2011; 10:626–636.
Article2. Rinkel GJ, Algra A. Long-term outcomes of patients with aneurysmal subarachnoid haemorrhage. Lancet Neurol. 2011; 10:349–356.
Article3. Clinical Research Center for Stroke. Clinical Practice Guideline for Stroke. 2nd ed. Seoul: Clinical Research Center for Stroke;2015.4. Jeong HW, Seo JH, Kim ST, Jung CK, Suh SI. Clinical practice guideline for the management of intracranial aneurysms. Neurointervention. 2014; 9:63–71.
Article5. Cherng TW, Jackson-Weaver O, Kanagy NL. Introduction to cardiovascular physiology. McQueen C. Comprehensive toxicology. 3rd ed. Oxford: Elsevier;2018. p. 29–45.6. Walmsley JG. Vascular smooth muscle orientation in curved branches and bifurcations of human cerebral arteries. J Microsc. 1983; 131(Pt 3):377–389.
Article7. Arribas SM, Hinek A, González MC. Elastic fibres and vascular structure in hypertension. Pharmacol Ther. 2006; 111:771–791.
Article8. Ratinov G. Extradural intracranial portion of carotid artery; a clinicopathologic study. Arch Neurol. 1964; 10:66–73.9. Wilkinson IM. The vertebral artery. Extracranial and intracranial structure. Arch Neurol. 1972; 27:392–396.10. Burton AC. Relation of structure to function of the tissues of the wall of blood vessels. Physiol Rev. 1954; 34:619–642.
Article11. Qiao Y, Anwar Z, Intrapiromkul J, Liu L, Zeiler SR, Leigh R, et al. Patterns and implications of intracranial arterial remodeling in stroke patients. Stroke. 2016; 47:434–440.
Article12. Zhang XJ, Gao BL, Hao WL, Wu SS, Zhang DH. Presence of anterior communicating artery aneurysm is associated with age, bifurcation angle, and vessel diameter. Stroke. 2018; 49:341–347.
Article13. Majesky MW. Developmental basis of vascular smooth muscle diversity. Arterioscler Thromb Vasc Biol. 2007; 27:1248–1258.
Article14. Etchevers HC, Vincent C, Le Douarin NM, Couly GF. The cephalic neural crest provides pericytes and smooth muscle cells to all blood vessels of the face and forebrain. Development. 2001; 128:1059–1068.
Article15. Norman PE, Powell JT. Site specificity of aneurysmal disease. Circulation. 2010; 121:560–568.
Article16. Guo DC, Papke CL, He R, Milewicz DM. Pathogenesis of thoracic and abdominal aortic aneurysms. Ann N Y Acad Sci. 2006; 1085:339–352.
Article17. Kuzmik GA, Feldman M, Tranquilli M, Rizzo JA, Johnson M, Elefteriades JA. Concurrent intracranial and thoracic aortic aneurysms. Am J Cardiol. 2010; 105:417–420.
Article18. Shin YW, Jung KH, Moon J, Lee ST, Lee SK, Chu K, et al. Site-specific relationship between intracranial aneurysm and aortic aneurysm. Stroke. 2015; 46:1993–1996.
Article19. Schievink WI, Raissi SS, Maya MM, Velebir A. Screening for intracranial aneurysms in patients with bicuspid aortic valve. Neurology. 2010; 74:1430–1433.
Article20. Schievink WI, Mokri B, Piepgras DG, Gittenberger-de Groot AC. Intracranial aneurysms and cervicocephalic arterial dissections associated with congenital heart disease. Neurosurgery. 1996; 39:685–689. discussion 689-690.
Article21. Schievink WI, Riedinger M, Maya MM. Frequency of incidental intracranial aneurysms in neurofibromatosis type 1. Am J Med Genet A. 2005; 134A:45–48.
Article22. Southerland AM, Meschia JF, Worrall BB. shared associations of nonatherosclerotic, large-vessel, cerebrovascular arteriopathies: considering intracranial aneurysms, cervical artery dissection, moyamoya disease and fibromuscular dysplasia. Curr Opin Neurol. 2013; 26:13–28.23. Shin YW, Jung KH, Kim JM, Cho YD, Lee ST, Chu K, et al. Echocardiographic evidence of innate aortopathy in the human intracranial aneurysm. PLoS One. 2014; 9:e100569.
Article24. Rodríguez C, Martínez-González J, Raposo B, Alcudia JF, Guadall A, Badimon L. Regulation of lysyl oxidase in vascular cells: lysyl oxidase as a new player in cardiovascular diseases. Cardiovasc Res. 2008; 79:7–13.25. Gacheru SN, Trackman PC, Shah MA, O’Gara CY, Spacciapoli P, Greenaway FT, et al. Structural and catalytic properties of copper in lysyl oxidase. J Biol Chem. 1990; 265:19022–19027.
Article26. Jung KH, Chu K, Lee ST, Shin YW, Lee KJ, Park DK, et al. Experimental induction of cerebral aneurysms by developmental low copper diet. J Neuropathol Exp Neurol. 2016; 75:455–463.
Article27. Lönnerdal B. Copper nutrition during infancy and childhood. Am J Clin Nutr. 1998; 67(5 Suppl):1046S–1053S.
Article28. Lönnerdal B, Bell JG, Keen CL. Copper absorption from human milk, cow’s milk, and infant formulas using a suckling rat model. Am J Clin Nutr. 1985; 42:836–844.
Article29. Baharoglu MI, Lauric A, Safain MG, Hippelheuser J, Wu C, Malek AM. Widening and high inclination of the middle cerebral artery bifurcation are associated with presence of aneurysms. Stroke. 2014; 45:2649–2655.
Article30. Metaxa E, Tremmel M, Natarajan SK, Xiang J, Paluch RA, Mandelbaum M, et al. Characterization of critical hemodynamics contributing to aneurysmal remodeling at the basilar terminus in a rabbit model. Stroke. 2010; 41:1774–1782.
Article31. Seshadhri S, Janiga G, Beuing O, Skalej M, Thevenin D. Impact of stents and flow diverters on hemodynamics in idealized aneurysm models. J Biomech Eng. 2011; 133:071005.
Article32. Can A, Du R. Association of hemodynamic factors with intracranial aneurysm formation and rupture: systematic review and meta-analysis. Neurosurgery. 2016; 78:510–520.33. Li M, Wang J, Liu J, Zhao C, Yang X. Hemodynamics in ruptured intracranial aneurysms with known rupture points. [published online ahead of print Jul 15, 2018] World Neurosurg 2018.
Article34. Zhou G, Zhu Y, Yin Y, Su M, Li M. Association of wall shear stress with intracranial aneurysm rupture: systematic review and meta-analysis. Sci Rep. 2017; 7:5331.
Article35. Can A, Castro VM, Ozdemir YH, Dagen S, Yu S, Dligach D, et al. Association of intracranial aneurysm rupture with smoking duration, intensity, and cessation. Neurology. 2017; 89:1408–1415.
Article36. Cho YD, Jung KH, Roh JK, Kang HS, Han MH, Lim JW. Characteristics of intracranial aneurysms associated with extracranial carotid artery disease in South Korea. Clin Neurol Neurosurg. 2013; 115:1677–1681.
Article37. Jou LD, Shaltoni HM, Morsi H, Mawad ME. Hemodynamic relationship between intracranial aneurysm and carotid stenosis: review of clinical cases and numerical analyses. Neurol Res. 2010; 32:1083–1089.
Article38. Chalouhi N, Hoh BL, Hasan D. Review of cerebral aneurysm formation, growth, and rupture. Stroke. 2013; 44:3613–3622.
Article39. Chalouhi N, Ali MS, Jabbour PM, Tjoumakaris SI, Gonzalez LF, Rosenwasser RH, et al. Biology of intracranial aneurysms: role of inflammation. J Cereb Blood Flow Metab. 2012; 32:1659–1676.
Article40. Aoki T, Nishimura M. Targeting chronic inflammation in cerebral aneurysms: focusing on NF-kappaB as a putative target of medical therapy. Expert Opin Ther Targets. 2010; 14:265–273.41. Fukuda M, Aoki T. Molecular basis for intracranial aneurysm formation. Acta Neurochir Suppl. 2015; 120:13–15.
Article42. Aoki T, Kataoka H, Shimamura M, Nakagami H, Wakayama K, Moriwaki T, et al. NF-kappaB is a key mediator of cerebral aneurysm formation. Circulation. 2007; 116:2830–2840.43. Aoki T, Nishimura M, Matsuoka T, Yamamoto K, Furuyashiki T, Kataoka H, et al. PGE(2) -EP(2) signalling in endothelium is activated by haemodynamic stress and induces cerebral aneurysm through an amplifying loop via NF-κB. Br J Pharmacol. 2011; 163:1237–1249.44. Fennell VS, Kalani MY, Atwal G, Martirosyan NL, Spetzler RF. Biology of saccular cerebral aneurysms: a review of current understanding and future directions. Front Surg. 2016; 3:43.
Article45. Chalouhi N, Points L, Pierce GL, Ballas Z, Jabbour P, Hasan D. Localized increase of chemokines in the lumen of human cerebral aneurysms. Stroke. 2013; 44:2594–2597.
Article46. Cao RY, Amand T, Ford MD, Piomelli U, Funk CD. The murine angiotensin ii-induced abdominal aortic aneurysm model: rupture risk and inflammatory progression patterns. Front Pharmacol. 2010; 1:9.
Article47. Aoki T, Frősen J, Fukuda M, Bando K, Shioi G, Tsuji K, et al. Prostaglandin E2-EP2-NF-κB signaling in macrophages as a potential therapeutic target for intracranial aneurysms. Sci Signal. 2017; 10:eaah6037.
Article48. Etminan N, Buchholz BA, Dreier R, Bruckner P, Torner JC, Steiger HJ, et al. Cerebral aneurysms: formation, progression, and developmental chronology. Transl Stroke Res. 2014; 5:167–173.
Article49. Wiebers DO, Whisnant JP, Huston J 3rd, Meissner I, Brown RD Jr, Piepgras DG, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003; 362:103–110.
Article50. UCAS Japan Investigators, Morita A, Kirino T, Hashi K, Aoki N, Fukuhara S, et al. The natural course of unruptured cerebral aneurysms in a japanese cohort. N Engl J Med. 2012; 366:2474–2482.
Article51. Molyneux AJ, Kerr RS, Yu LM, Clarke M, Sneade M, Yarnold JA, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005; 366:809–817.
Article52. Koroknay-Pál P, Niemelä M, Lehto H, Kivisaari R, Numminen J, Laakso A, et al. De novo and recurrent aneurysms in pediatric patients with cerebral aneurysms. Stroke. 2013; 44:1436–1439.
Article53. Bor AS, Tiel Groenestege AT, terBrugge KG, Agid R, Velthuis BK, Rinkel GJ, et al. Clinical, radiological, and flow-related risk factors for growth of untreated, unruptured intracranial aneurysms. Stroke. 2015; 46:42–48.
Article54. Backes D, Vergouwen MD, Tiel Groenestege AT, Bor AS, Velthuis BK, Greving JP, et al. PHASES score for prediction of intracranial aneurysm growth. Stroke. 2015; 46:1221–1226.
Article55. Matsubara S, Hadeishi H, Suzuki A, Yasui N, Nishimura H. Incidence and risk factors for the growth of unruptured cerebral aneurysms: observation using serial computerized tomography angiography. J Neurosurg. 2004; 101:908–914.
Article56. Zhou S, Dion PA, Rouleau GA. Genetics of intracranial aneurysms. Stroke. 2018; 49:780–787.
Article57. Nurmonen HJ, Huttunen T, Huttunen J, Kurki MI, Helin K, Koivisto T, et al. Polycystic kidney disease among 4,436 intracranial aneurysm patients from a defined population. Neurology. 2017; 89:1852–1859.
Article58. Lather HD, Gornik HL, Olin JW, Gu X, Heidt ST, Kim ESH, et al. Prevalence of intracranial aneurysm in women with fibromuscular dysplasia: a report from the us registry for fibromuscular dysplasia. JAMA Neurol. 2017; 74:1081–1087.59. Connolly HM, Huston J 3rd, Brown RD Jr, Warnes CA, Ammash NM, Tajik AJ. Intracranial aneurysms in patients with coarctation of the aorta: a prospective magnetic resonance angiographic study of 100 patients. Mayo Clin Proc. 2003; 78:1491–1499.
Article60. Rosenquist TH, Beall AC, Módis L, Fishman R. Impaired elastic matrix development in the great arteries after ablation of the cardiac neural crest. Anat Rec. 1990; 226:347–359.
Article61. Vlak MH, Rinkel GJ, Greebe P, van der Bom JG, Algra A. Trigger factors and their attributable risk for rupture of intracranial aneurysms: a case-crossover study. Stroke. 2011; 42:1878–1882.62. Galea E, Santizo R, Feinstein DL, Adamsom P, Greenwood J, Koenig HM, et al. Estrogen inhibits NF kappa B-dependent inflammation in brain endothelium without interfering with I kappa B degradation. Neuroreport. 2002; 13:1469–1472.63. Chambliss KL, Shaul PW. Estrogen modulation of endothelial nitric oxide synthase. Endocr Rev. 2002; 23:665–686.
Article64. Strehlow K, Rotter S, Wassmann S, Adam O, Grohé C, Laufs K, et al. Modulation of antioxidant enzyme expression and function by estrogen. Circ Res. 2003; 93:170–177.
Article65. Nguyen TV, Jones G, Sambrook PN, White CP, Kelly PJ, Eisman JA. Effects of estrogen exposure and reproductive factors on bone mineral density and osteoporotic fractures. J Clin Endocrinol Metab. 1995; 80:2709–2714.
Article66. Almeida M, Laurent MR, Dubois V, Claessens F, O’Brien CA, Bouillon R, et al. Estrogens and androgens in skeletal physiology and pathophysiology. Physiol Rev. 2017; 97:135–187.
Article67. Shin YW, Park KI, Moon J, Lee ST, Chu K, Lee SK, et al. Association of bone mineral density with the risk of intracranial aneurysm. JAMA Neurol. 2018; 75:179–186.
Article68. Dupin E, Sommer L. Neural crest progenitors and stem cells: from early development to adulthood. Dev Biol. 2012; 366:83–95.
Article69. Viguet-Carrin S, Garnero P, Delmas PD. The role of collagen in bone strength. Osteoporos Int. 2006; 17:319–336.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Superior Temporal Gyrus Approach to Middle Cerebral Artery Aneurysms
- Multiple Aneurysms of the Distal Anterior Cerebral Artery: Case Report
- The Clinical Usefulness and the Value of 3D CT Angiography using Spiral CT in Patients with Cerebral Aneurysms
- Surgical Treatment of Unruptured Cerebral Aneurysms
- Moyamoya Disease Associated with Aneurysm