J Korean Orthop Assoc.
1996 Apr;31(2):247-254. 10.4055/jkoa.1996.31.2.247.
Effect of Dehydroepiandrosterone on the Osteoporosis Induced by Oophorectomy in the Rat
- KMID: 2423601
- DOI: http://doi.org/10.4055/jkoa.1996.31.2.247
Abstract
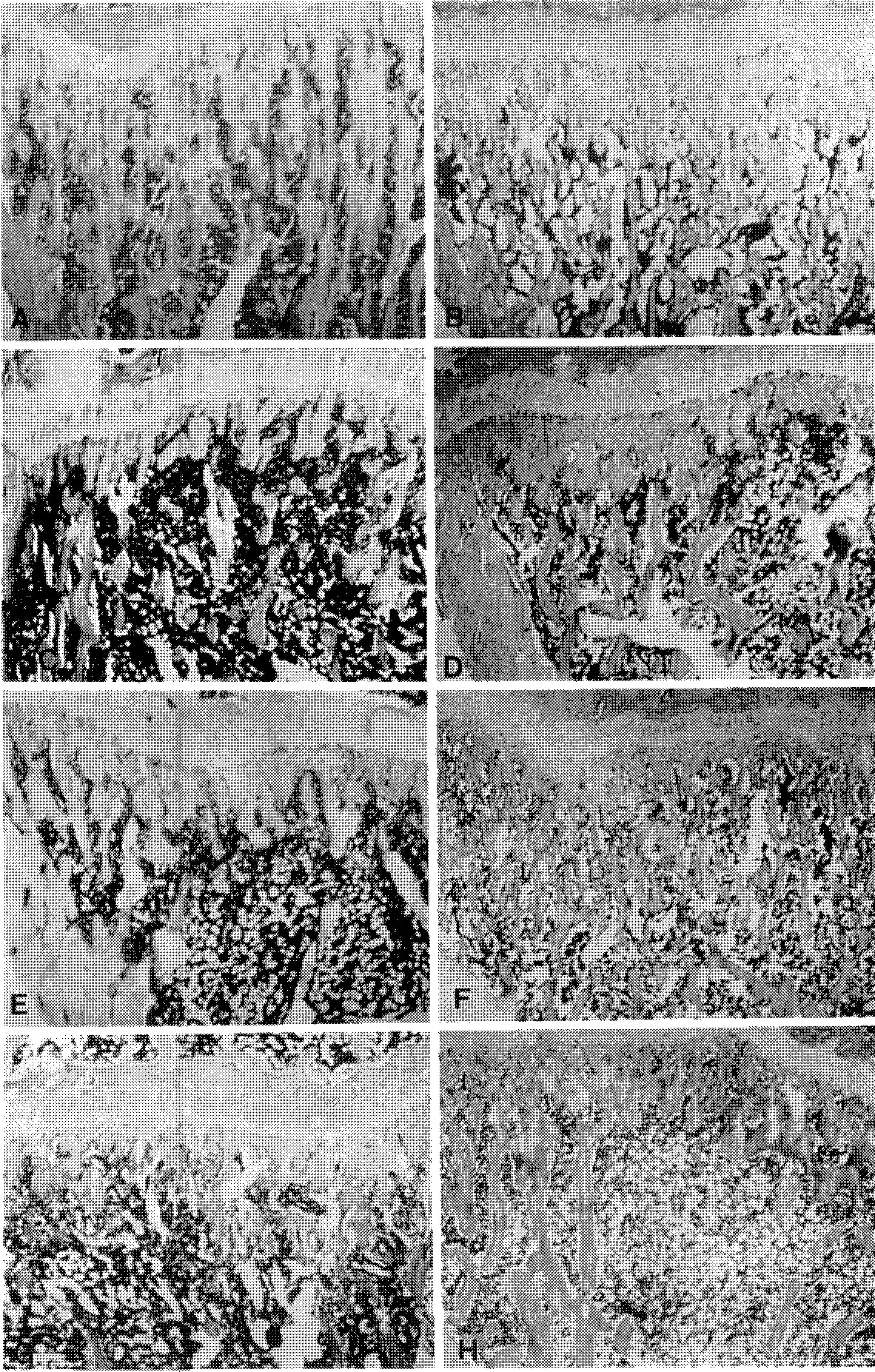
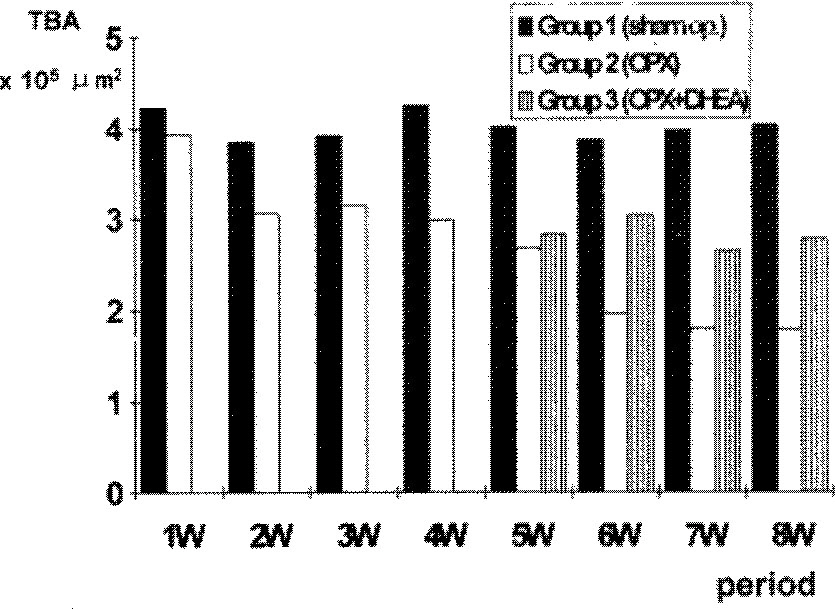
- Dehydroepiandrosterone(DHEA) was administered to rats to investigate the efficacy of DHEA in prevention of the process of osteoporosis induced by oophorectomy. Female Sprague-Dawley rats, aged five months, were divided into three groups; Group 1(40 rats), sham operation as control; group 2(40 rats), bilateral oophorectomy; and group 3(20 rats), intraperitoneal injection of 10mg of DHEA every other day from 4 weeks after bilateral oophorectomy. In group 1 and 2, five rats of each group were sacrificed weekly until the 8th week. In group 3, five rats were killed weekly from 1 week to 4 weeks after DHEA administration. Trabecular bone area(TBA) of proximal metaphysic of left tibia was obtained by quantitative image analysis system. In group 1, TBA was not changed with time after sham operation, In group 2, TBA decreased progressively through eight weeks after oophorectomy. In group 3, TBA's were higher than those of group 2, but they were still lower than those of group 1. These data suggested that DHEA, administered to rats from 4 weeks to 8 weeks after bilateral oophorectomy, may partially recover the osteoporosis or delay the progression of the osteoporotic change until 8 weeks after the oophorectomy.
Keyword
MeSH Terms
Figure
Reference
-
1. Arnaud CD. An Integrated View of the Role of the Endocrine System in the Genesis of the Osteoporosis Associated with Aging. Osteoporosis International. 1993; 3(suppl.1):37–39,.
Article2. Daynes RA, Araneo BA, Ershler WB, Maloney C, Li GZ, Ryu SY. Altered Regulation of IL-6 Production with Normal Aging. Possible Linkage to the Age-Associated Decline in Dehydroepiandrosterone and its Sulfated Derivative. Journal of Immunology. 1993; 150(12):5219–5230.3. Durbridge TC, Morris HA, Parson AM, Parkinson IH, Moore RJ, Porter S, Need AG, Nordin BEC, Vernon-Roberts B. Progressive Cancellous Bone Loss in Rats after Adrenaectomy and Oophorec-tomy. Calcified Tissue International. 1990; 47:383–387.4. Eriksen ER, Colvard PS, Berg NJ, Graham ML, Mann KG, Speltberg TC, Riggs BL. Evidence of Estrogen Receptor in Normal Human Osteoblast-Like Cell. Science. 1981; 241:84–86.5. Ershler WB, Sun WH, Binkley N, Volk MJ, Gravenstein S, Kamoske G, Klopp RG, Roecker EB, Daynes RA, Welndruch R. Interleukin-6 and Aging. Blood Levels and Mononuclear Cell Production Increase with Advancing Age and In Vitro Production Is Modifiable by Dietary Restriction. Lymphokine and Cytokine Research. 1993; 12({4)):225–23Q.6. Girasole G, Jilka RL, Passeri G, Boswell S, Boder G, Willams DC, Manolagas SC. 17β-Estradiol Inhibits Interleukin-6 Production by Bone Marrow-derived Stromal Cells and Osteoblasts in Vitro. Journal of Clinical Investigation. 1992; 89:883–891.7. Grkan L, Ekel A, Gautvik KM, Langeland N, Ronningen H, Solheim LF. Bone changes after Castration in Rats. A Model for Osteoporosis. Acta Orthopedica Scandinavia,. 1986; 57:67–70.8. Jayo MJ, Weaver DS, Adams MR, Rankin SE. Effects on Bone of Surgical Menopause and Estrogen Therapy with or without Progesteron Replacement in Cynomolgus Monkeys. Am J Obstet Gynecol. 1990; 193:614–618.9. Jilka RL, Hangoc G, Girasole G, Passeri G, Willams PCA, brains JS, Boyce B, Broxmeyer H, Manolagas SC. Increased Osteoclast Development after Estrogen Loss. Mediation by Interleukin-6. Science. 1992; 257:88–91.
Article10. Kalu DN, Liu CC, Hardin RR, Mollis BW. The Aged Rat Model of Ovarian Hormone Deficiency Bone Loss. Endocrinology. 1989; 124:7–16.
Article11. Lindgren U, DeLuca HF. Role of Parathyroid Hormone and 1,25-Dihydroxyvitamin D3 in the Development of Osteopenia in Oophoreetomized Rats. Calcified Tissue International. 1982; 34:510–514.12. Lindsay R. Estrogen Therapy in the Prevention and Management of Osteoporosis. Am J Obstet Gynecol. 1987; 156:1347–1351.
Article13. Malluehe HH, Sherman D, Meyer W, Massry SG. A New Semiautomatic Method for Quantitative Static and Dynamic Bone Histology. Calcified Tissue International. 1982; 34:439–448.14. Onto H, Masuzawa T, Ikeda T, Suda Y, Makita K, Nozawa S. Which is More Osteoporosis-inducing Menopause or Oophorectomy? Bone and Mineral. 1992; 19((3)):273–285.
Article15. Orimo H, Fujita T, Yoshikawa M. Increased Sensitivity of Bone to Parathyroid Hormone in Ovariectomized Rats. Endocrinology. 1972; 90:760–763.
Article16. Recker RR, Saville PD, Heaney RP. Effect of Estrogen and Calcium Carbonate on Bone Loss in Postmenopausal Women. Annals of Internal Medicine. 1977; 87:649–655.17. Schlaghccke R, Kley HK, Juli E. Age-related Changes in 11β-hydroxyandrostenedione Concentration in Normal and Osteoporotic Women. Journal of Steroid Biochemistry and Molecular Biology. 40:1991; ((4-6)):731–733.18. Weksler ME. Immune Senescence and Adrenal Steroids. Immune dysregulation and the Action of Dehydroepinadrosterone(DHEA) in Old Animals. European Journal of Clinical Pharmacology,. 1993; 45(Suppl 1):S21–23. Discussion S43-44.19. Wild RA, Buchanan JR, Myers C, Demers LM. Declining Adrenal Androgen. an Association with Bone Loss in Aging Women. Proceedings of the Society for Experimental Biology and Medicine,. 1987; 186((3)):355–360.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Estradiol on the Osteoporosis Induced by Oophorectomy in the Rat
- DHEA ( dehydroepiandrosterone )
- Comparison of Anti-osteoporotic Medications and Alternatives
- Effects of dexamethasone and DHEA on the responses of rat cerebral cortical astrocytes to lipopolysaccharide and antimycin A
- Effect of calcitonin, NaF and tamoxifen on osteoporosis induced by ovariectomy in rat