Korean J Schizophr Res.
2018 Oct;21(2):43-50. 10.16946/kjsr.2018.21.2.43.
Association between a Genetic Variant of CACNA1C and the Risk of Schizophrenia and Bipolar I Disorder Across Diagnostic Boundaries
- Affiliations
-
- 1Department of Psychiatry, Sungkyunkwan University School of Medicine, Samsung Medical Center, Seoul, Korea. hongks@skku.edu
- 2Center for Clinical Research, Samsung Biomedical Research Institute, Seoul, Korea.
- 3St. Andrew's Hospital, Icheon, Korea.
- 4Yong-In Mental Hospital, Yongin, Korea.
- 5Department of Psychiatry, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2423026
- DOI: http://doi.org/10.16946/kjsr.2018.21.2.43
Abstract
OBJECTIVES
Genome-wide association studies (GWASs) and meta-analyses indicate that single-nucleotide polymorphisms (SNPs) in the a-1C subunit of the L-type voltage-dependent calcium channel (CACNA1C) gene increase the risk for schizophrenia and bipolar disorders (BDs). We investigated the association between the genetic variants on CACNA1C and schizophrenia and/or BDs in the Korean population.
METHODS
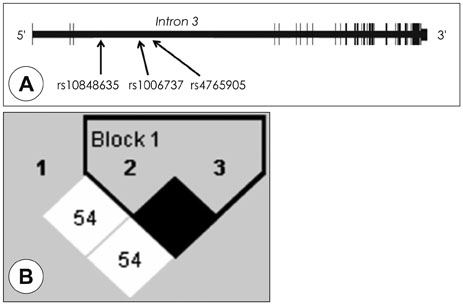
A total of 582 patients with schizophrenia, 336 patients with BDs consisting of 179 bipolar I disorder (BD-I) and 157 bipolar II disorder (BD-II), and 502 healthy controls were recruited. Based on previous results from other populations, three SNPs (rs10848635, rs1006737, and rs4765905) were selected and genotype-wise association was evaluated using logistic regression analysis under additive, dominant and recessive genetic models.
RESULTS
rs10848635 showed a significant association with schizophrenia (p=0.010), the combined schizophrenia and BD group (p=0.018), and the combined schizophrenia and BD-I group (p=0.011). The best fit model was dominant model for all of these phenotypes. The association remained significant after correction for multiple testing in schizophrenia and the combined schizophrenia and BD-I group.
CONCLUSION
We identified a possible role of CACNA1C in the common susceptibility of schizophrenia and BD-I. However no association trend was observed for BD-II. Further efforts are needed to identify a specific phenotype associated with this gene crossing the current diagnostic categories.
MeSH Terms
Figure
Reference
-
1. Craddock N, Owen MJ. The beginning of the end for the Kraepelinian dichotomy. Br J Psychiatry. 2005; 186:364–366.
Article2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorder: DSM-5. 5th ed. Washington, DC: American Psychiatric Publishing;2013.3. Leboyer M, Schurhoff F. Searching across diagnostic boundaries. Schizophr Bull. 2014; 40:946–948.
Article4. Lichtenstein P, Yip BH, Bjork C, Pawitan Y, Cannon TD, Sullivan PF, et al. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009; 373:234–239.
Article5. Tamminga CA, Ivleva EI, Keshavan MS, Pearlson GD, Clementz BA, Witte B, et al. Clinical phenotypes of psychosis in the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP). Am J Psychiatry. 2013; 170:1263–1274.
Article6. Valles V, Van Os J, Guillamat R, Gutierrez B, Campillo M, Gento P, et al. Increased morbid risk for schizophrenia in families of in-patients with bipolar illness. Schizophr Res. 2000; 42:83–90.
Article7. Moon AL, Haan N, Wilkinson LS, Thomas KL, Hall J. CACNA1C: Association With Psychiatric Disorders, Behavior, and Neurogenesis. Schizophr Bull. 2018; 44:958–965.
Article8. Ortner NJ, Striessnig J. L-type calcium channels as drug targets in CNS disorders. Channels (Austin). 2016; 10:7–13.
Article9. Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron. 1998; 20:709–726.
Article10. Tao X, West AE, Chen WG, Corfas G, Greenberg ME. A calcium-responsive transcription factor, CaRF, that regulates neuronal activity-dependent expression of BDNF. Neuron. 2002; 33:383–395.
Article11. Wheeler DG, Groth RD, Ma H, Barrett CF, Owen SF, Safa P, et al. Ca(V)1 and Ca(V)2 channels engage distinct modes of Ca(2+) signaling to control CREB-dependent gene expression. Cell. 2012; 149:1112–1124.
Article12. Bhat S, Dao DT, Terrillion CE, Arad M, Smith RJ, Soldatov NM, et al. CACNA1C (Cav1.2) in the pathophysiology of psychiatric disease. Prog Neurobiol. 2012; 99:1–14.
Article13. Degoulet M, Stelly CE, Ahn KC, Morikawa H. L-type Ca(2)(+) channel blockade with antihypertensive medication disrupts VTA synaptic plasticity and drug-associated contextual memory. Mol Psychiatry. 2016; 21:394–402.
Article14. Weisskopf MG, Bauer EP, LeDoux JE. L-type voltage-gated calcium channels mediate NMDA-independent associative long-term potentiation at thalamic input synapses to the amygdala. J Neurosci. 1999; 19:10512–10519.
Article15. Ferreira MA, O'Donovan MC, Meng YA, Jones IR, Ruderfer DM, Jones L, et al. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat Genet. 2008; 40:1056–1058.
Article16. Sklar P, Smoller JW, Fan J, Ferreira MA, Perlis RH, Chambert K, et al. Whole-genome association study of bipolar disorder. Mol Psychiatry. 2008; 13:558–569.
Article17. Green EK, Grozeva D, Jones I, Jones L, Kirov G, Caesar S, et al. The bipolar disorder risk allele at CACNA1C also confers risk of recurrent major depression and of schizophrenia. Mol Psychiatry. 2010; 15:1016–1022.
Article18. Schizophrenia Working Group of the Psychiatric Genomics Consortium. Ripke S, Neale B, Corvin A. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014; 511:421–427.
Article19. Takahashi S, Glatt SJ, Uchiyama M, Faraone SV, Tsuang MT. Meta-analysis of data from the Psychiatric Genomics Consortium and additional samples supports association of CACNA1C with risk for schizophrenia. Schizophr Res. 2015; 168:429–433.
Article20. Porcelli S, Lee SJ, Han C, Patkar AA, Serretti A, Pae CU. CACNA1C gene and schizophrenia: a case-control and pharmacogenetic study. Psychiatr Genet. 2015; 25:163–167.21. Jan WC, Yang SY, Chuang LC, Lu RB, Lu MK, Sun HS, et al. Exploring the associations between genetic variants in genes encoding for subunits of calcium channel and subtypes of bipolar disorder. J Affect Disord. 2014; 157:80–86.
Article22. He K, An Z, Wang Q, Li T, Li Z, Chen J, et al. CACNA1C, schizophrenia and major depressive disorder in the Han Chinese population. Br J Psychiatry. 2014; 204:36–39.
Article23. Hamshere ML, Walters JT, Smith R, Richards AL, Green E, Grozeva D, et al. Genome-wide significant associations in schizophrenia to ITIH3/4, CACNA1C and SDCCAG8, and extensive replication of associations reported by the Schizophrenia PGC. Mol Psychiatry. 2013; 18:708–712.
Article24. Green EK, Hamshere M, Forty L, Gordon-Smith K, Fraser C, Russell E, et al. Replication of bipolar disorder susceptibility alleles and identification of two novel genome-wide significant associations in a new bipolar disorder case-control sample. Mol Psychiatry. 2013; 18:1302–1307.
Article25. Gonzalez S, Xu C, Ramirez M, Zavala J, Armas R, Contreras SA, et al. Suggestive evidence for association between L-type voltage-gated calcium channel (CACNA1C) gene haplotypes and bipolar disorder in Latinos: a family-based association study. Bipolar Disord. 2013; 15:206–214.
Article26. Nyegaard M, Demontis D, Foldager L, Hedemand A, Flint TJ, Sorensen KM, et al. CACNA1C (rs1006737) is associated with schizophrenia. Mol Psychiatry. 2010; 15:119–121.
Article27. Casamassima F, Huang J, Fava M, Sachs GS, Smoller JW, Cassano GB, et al. Phenotypic effects of a bipolar liability gene among individuals with major depressive disorder. Am J Med Genet B Neuropsychiatr Genet. 2010; 153b:303–309.
Article28. Liu Y, Blackwood DH, Caesar S, de Geus EJ, Farmer A, Ferreira MA, et al. Meta-analysis of genome-wide association data of bipolar disorder and major depressive disorder. Mol Psychiatry. 2011; 16:2–4.
Article29. Li J, Zhao L, You Y, Lu T, Jia M, Yu H, et al. Schizophrenia Related Variants in CACNA1C also Confer Risk of Autism. PLoS One. 2015; 10:e0133247.
Article30. Judd LL, Akiskal HS, Schettler PJ, Coryell W, Maser J, Rice JA, et al. The comparative clinical phenotype and long term longitudinal episode course of bipolar I and II: a clinical spectrum or distinct disorders? J Affect Disord. 2003; 73:19–32.
Article31. Judd LL, Schettler PJ, Akiskal HS, Maser J, Coryell W, Solomon D, et al. Long-term symptomatic status of bipolar I vs. bipolar II disorders. Int J Neuropsychopharmacol. 2003; 6:127–137.
Article32. Kim JS, Ha TH, Chang JS, Park YS, Huh I, Kim J, et al. Seasonality and its distinct clinical correlates in bipolar II disorder. Psychiatry Res. 2015; 225:540–544.
Article33. Vieta E, Gasto C, Otero A, Nieto E, Vallejo J. Differential features between bipolar I and bipolar II disorder. Compr Psychiatry. 1997; 38:98–101.
Article34. Baek JH, Park DY, Choi J, Kim JS, Choi JS, Ha K, et al. Differences between bipolar I and bipolar II disorders in clinical features, comorbidity, and family history. J Affect Disord. 2011; 131:59–67.
Article35. Choi J, Baek JH, Noh J, Kim JS, Choi JS, Ha K, et al. Association of seasonality and premenstrual symptoms in bipolar I and bipolar II disorders. J Affect Disord. 2011; 129:313–316.
Article36. Merikangas KR, He JP, Burstein M, Swanson SA, Avenevoli S, Cui L, et al. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication--Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry. 2010; 49:980–989.
Article37. Moreno-Kustner B, Martin C, Pastor L. Prevalence of psychotic disorders and its association with methodological issues. A systematic review and meta-analyses. PLoS One. 2018; 13:e0195687.
Article38. Cross-Disorder Group of the Psychiatric Genomics Consortium. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013; 381:1371–1379.39. Cocchi E, Fabbri C, Han C, Lee SJ, Patkar AA, Masand PS, et al. Genome-wide association study of antidepressant response: involvement of the inorganic cation transmembrane transporter activity pathway. BMC Psychiatry. 2016; 16:106.
Article40. Calabro M, Mandelli L, Crisafulli C, Sidoti A, Jun TY, Lee SJ, et al. Genes Involved in Neurodevelopment, Neuroplasticity, and Bipolar Disorder: CACNA1C, CHRNA1, and MAPK1. Neuropsychobiology. 2016; 74:159–168.
Article41. Lett TA, Zai CC, Tiwari AK, Shaikh SA, Likhodi O, Kennedy JL, et al. ANK3, CACNA1C and ZNF804A gene variants in bipolar disorders and psychosis subphenotype. World J Biol Psychiatry. 2011; 12:392–397.
Article42. Ruderfer DM, Fanous AH, Ripke S, McQuillin A, Amdur RL, Gejman PV, et al. Polygenic dissection of diagnosis and clinical dimensions of bipolar disorder and schizophrenia. Mol Psychiatry. 2014; 19:1017–1024.
Article43. Merikangas KR, Cui L, Heaton L, Nakamura E, Roca C, Ding J, et al. Independence of familial transmission of mania and depression: results of the NIMH family study of affective spectrum disorders. Mol Psychiatry. 2014; 19:214–219.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Association of CACNA1C Variants with Bipolar Disorder in the Korean Population
- Genome-Wide Supported Risk Variants in MIR137, CACNA1C, CSMD1, DRD2, and GRM3 Contribute to Schizophrenia Susceptibility in Pakistani Population
- The Frequencies of HLA Alleles in Bipolar Disorder
- Comparison of P300 between Schizophrenia and Bipolar Disorder
- Shared and Distinct Neurocognitive Endophenotypes of Schizophrenia and Psychotic Bipolar Disorder