Nat Prod Sci.
2018 Sep;24(3):171-180. 10.20307/nps.2018.24.3.171.
Anti-inflammatory Potential of Artemisia capillaris and Its Constituents in LPS-induced RAW264.7 Cells
- Affiliations
-
- 1Department of Food and Life Science, Pukyong National University, Busan 48513, Republic of Korea. choijs@pknu.ac.kr
- 2Department of Pharmacy, Mawlana Bhashani Science and Technology University, Santosh, Tangail 1902, Bangladesh.
- 3Department of Food Science and Human Nutrition, Chonbuk National University, Jeonju 54896, Republic of Korea. jungha@jbnu.ac.kr
- KMID: 2422009
- DOI: http://doi.org/10.20307/nps.2018.24.3.171
Abstract
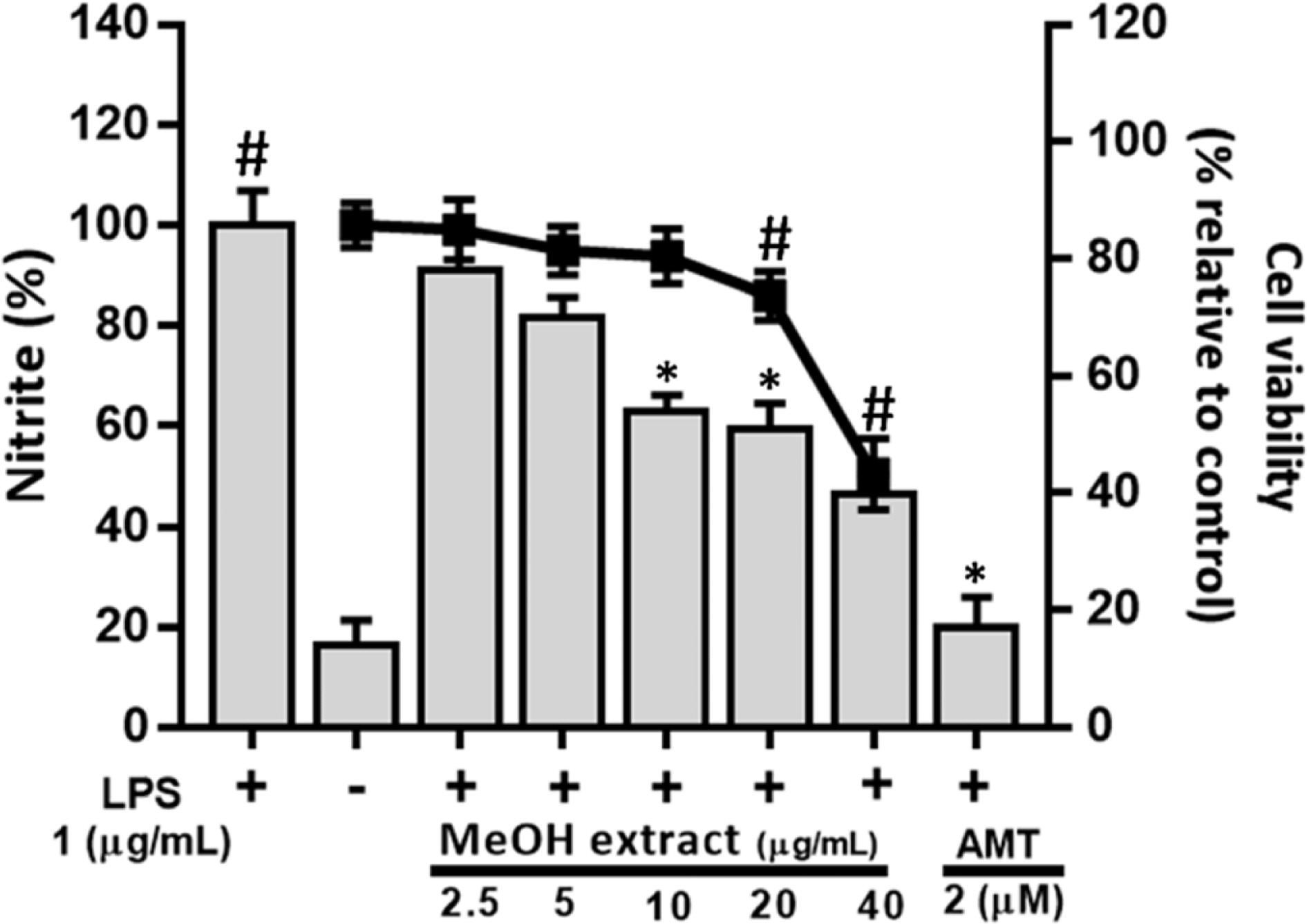
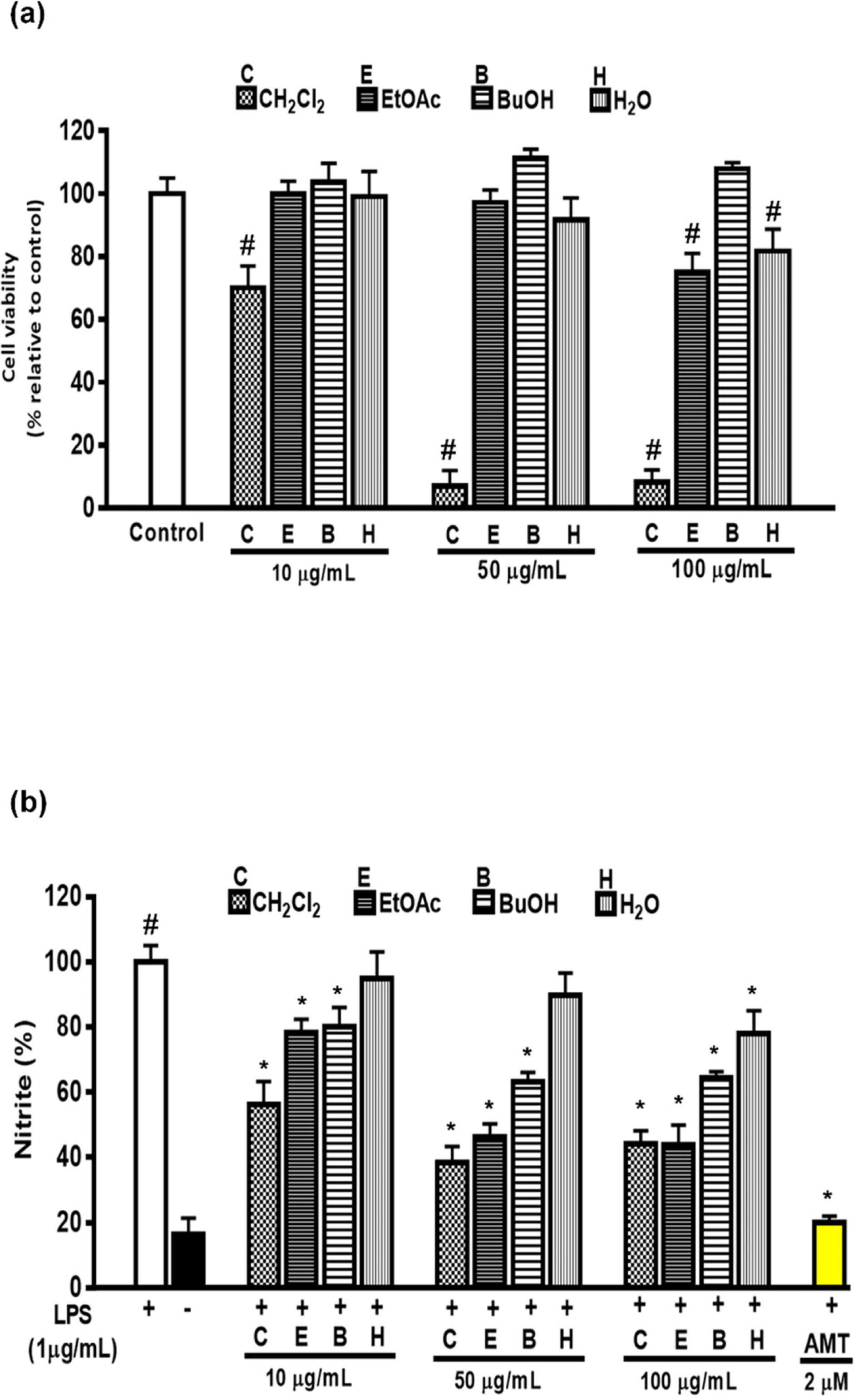
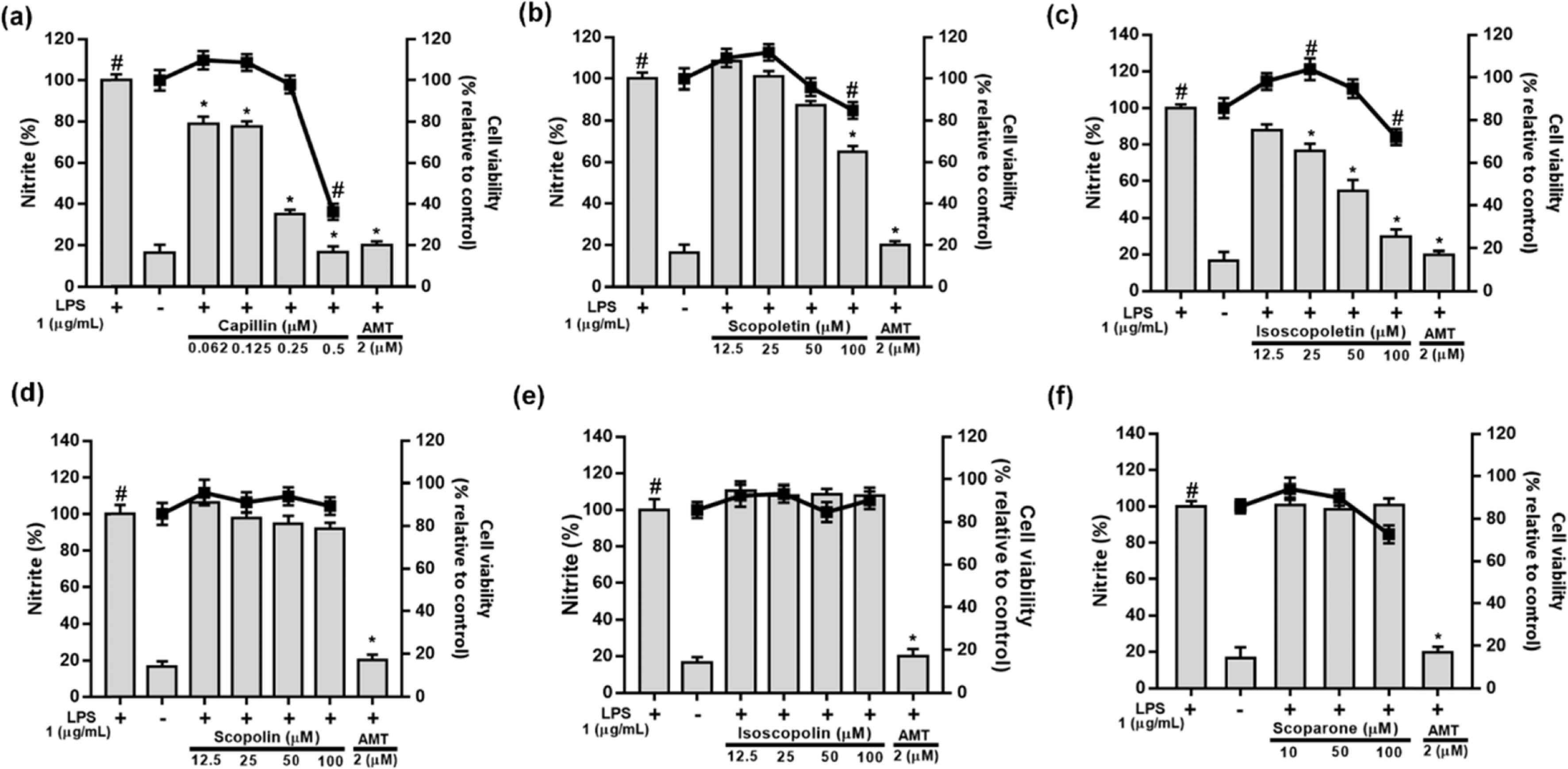
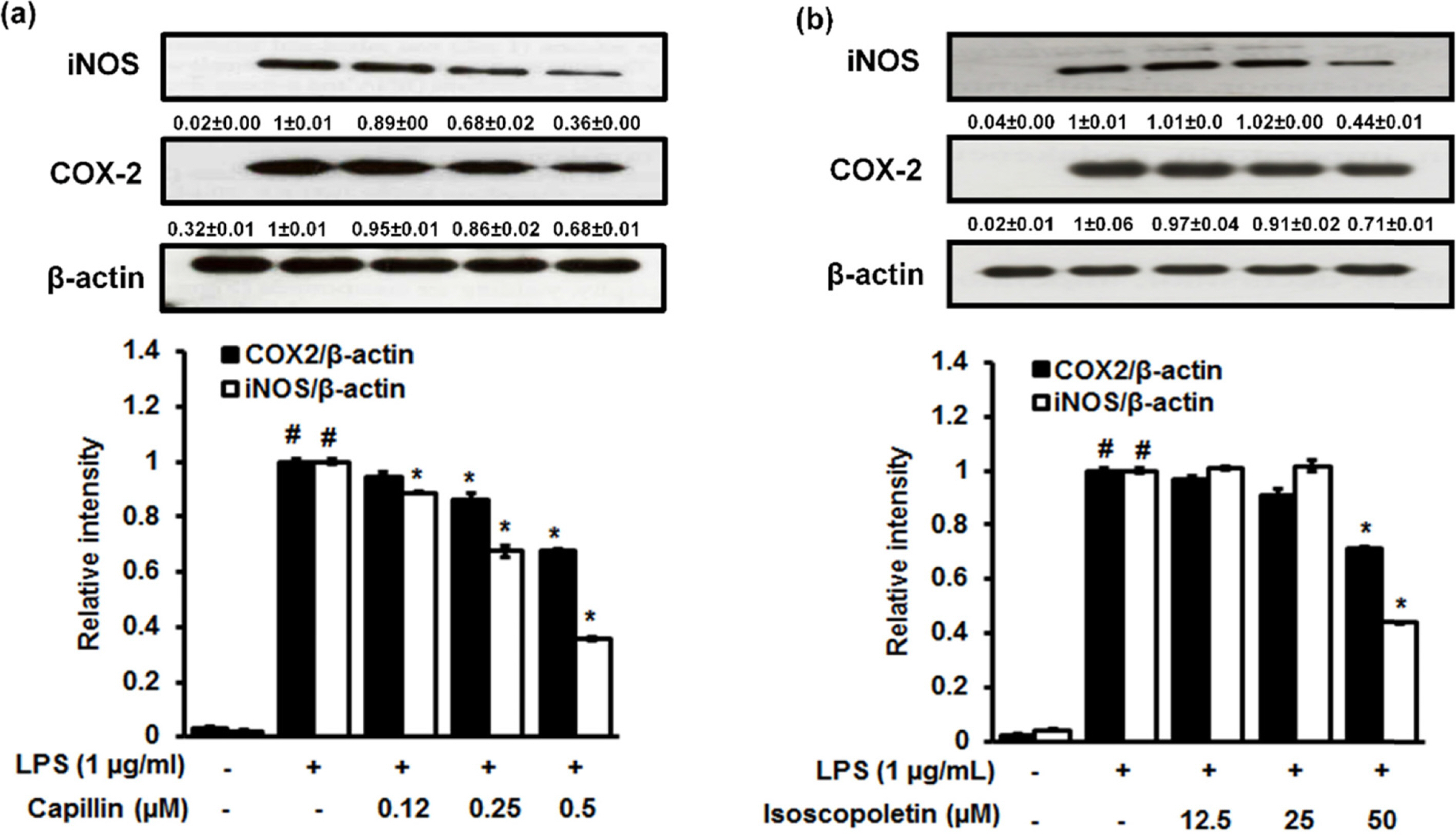
- Artemisia capillaris has been widely used as an alternative therapy for treating obesity and atopic dermatitis. It has been used as a hepatoprotactant. It is also used for ameliorating inflammatory reactions. Although there are several investigations on other Artemisia species, there is no systematic study describing the role of A. capillaris MeOH extract, its solvent soluble fractions, or derived anti-inflammatory principal components in regulating inflammatory conditions. Therefore, the objective of this study was to elucidate anti-inflammatory mechanisms of A. capillaris. Results revealed that MeOH extract of A. capillaris could decrease LPS-stimulated NO secretion. Of tested fractions, CHâ‚‚Clâ‚‚, EtOAc, and n-BuOH strongly inhibited NO release from RAW264.7 cells. Bioactive mediators derived from CHâ‚‚Clâ‚‚ and n-BuOH fractions elicited potent anti-inflammatory actions and strikingly abrogated LPS-triggered NO accumulation in RAW264.7 cells. Of particular interest, capillin and isoscopoletin possessed the most potent NO suppressive effects. Western blot analysis validated the molecular mechanism of NO inhibition and showed that capillin and isoscopoletin significantly down-regulated iNOS and COX-2 protein expression. Taken together, our results provide the first evidence that MeOH extract, CHâ‚‚Clâ‚‚, EtOAc, and n-BuOH fractions from A. capillaris and its derived lead candidates can potently suppress inflammatory responses in macrophages by hampering NO release and down-regulating iNOS and COX-2 signaling.
Keyword
MeSH Terms
Figure
Reference
-
(1). Murakami M., Hirano T.Front. Immunol. 2012; 3:1–2.(2). Sharma J. N., Al-Omran A., Parvathy S. S.Inflammopharmacology. 2007; 15:252–259.(3). Bognar E., Sarszegi Z., Szabo A., Debreceni B., Kalman N., Tucsek Z., Sumegi B., Gallyas F.Jr. PLoS One. 2013; 8:e65355.(4). Rhule A., Navarro S., Smith J. R., Shepherd D. M. J.Ethnopharmacol. 2006; 106:121–128.(5). Tang W., Eisenbrand G.Chinese drugs of plant origin, chemistry, phamacology and use in traditional and modern medicine: Springer Verlag, New York. 1992; 179.(6). Hong J. H., Lee J. W., Park J. H., Lee I. S.Biofactors. 2007; 31:43–53.(7). Seo K. S., Yun K. W.Korean J. Plant Res. 2008; 21:292–298.(8). Lee C. J., Kim H. Y., Kim J. D., Kim C. H. J.Korean Orient. Med. 2000; 21:100–107.(9). Choi J. H., Kim D. W., Yun N., Choi J. S., Islam M. N., Kim Y. S., Lee S. M. J.Nat. Prod. 2011; 74:1055–1060.(10). Seo K. S., Jeong H. J., Yun K. W. J.Ecol. Field Biol. 2010; 33:141–147.(11). Cha J. D., Moon S. E., Kim H. Y., Cha I. H., Lee K. Y. J.Food Sci. 2009; 74:75–81.(12). Hong J. H., Lee I. S.Biofactors. 2009; 35:380–388.
Article(13). Okuno I., Uchida K., Kadowaki M., Akahori A. Jpn. J.Pharmacol. 1981; 31:835–838.(14). Jung H. A., Park J. J., Islam M. N., Jin S. E., Min B. S., Lee J. H., Sohn H. S., Choi J. S.Arch. Pharm. Res. 2012; 35:1021–1035.(15). Kwon O. S., Choi J. S., Islam M. N., Kim Y. S., Kim H. P.Arch. Pharm. Res. 2011; 34:1561–1569.(16). Nurul Islam M., Jung H. A., Sohn H. S., Kim H. M., Choi J. S.Arch. Pharm. Res. 2013; 36:542–552.(17). Pautz A., Art J., Hahn S., Nowag S., Voss C., Kleinert H.Nitric Oxide. 2010; 23:75–93.(18). Ribiere C., Jaubert A. M., Gaudiot N., Sabourault D., Marcus M. L., Boucher J. L., Denis-Henriot D., Giudicelli Y.Biochem. Biophys. Res. Commun. 1996; 222:706–712.(19). Mitrovic B., Ignarro L. J., Montestruque S., Smoll A., Merrill J. E.Neuroscience. 1994; 61:575–585.(20). Kawachi H., Moriya N. H., Korai T., Tanaka S. Y., Watanabe M., Matsui T., Kawada T., Yano H.Mol. Cell. Biochem. 2007; 300:61–67.67.(21) Rayburn E. R.., Ezell S. J.., Zhang R.Mol. Cell Pharmacol. 2009. 1:29–43.(22). Mulabagal V., Alexander-Lindo R. L., Dewitt D. L., Nair M. G.Evid. Based Complement. Alternat. Med. 2011; 11:1–6.(23). Yan Y., Li J., Ouyang W., Ma Q., Hu Y., Zhang D., Ding J., Qu Q., Subbaramaiah K., Huang C. J.Cell Sci. 2006; 119:2985–2994.(24). Turini M. E., DuBois R. N.Annu. Rev. Med. 2002; 53:35–57.
Article(25). Burk D. R., Cichacz Z. A., Daskalova S. M. J.Med. Plant. Res. 2010; 4:225–234.(26). Valles J., Torrell M., Garnatje T., Garcia-Jacas N., Vilatersana R., Susanna A.Plant Biol. 2003; 5:274–284.(27). Lim H. K., Cho S. K., Park S., Cho M. J.Korean Soc. Appl. Biol. Chem. 2010; 53:275–282.(28). Jang M., Jeong S. W., Kim B. K., Kim J. C.BioMed. Res. Int. 2015; 2015:872718.(29). Ali M. Y., Jannat S., Jung H. A., Choi R. J., Roy A., Choi J. S. Asian Pac. J.Trop. Med. 2016; 9:103–111.(30). Han J., Zhao Y. L., Shan L. M., Huang F. J., Xiao X. H. Chin. J.Integr. Med. 2005; 11:54–56.(31). Kim E. K., Kwon K. B., Han M. J., Song M. Y., Lee J. H., Lv N., Choi K. B., Ryu D. G., Kim K. S., Park J. W., Park B. H.Int. J. Mol. Med. 2007; 19:535–540.(32). Kim Y. S., Bahn K. N., Hah C. K., Gang H. I., Ha Y. L.J. Food Sci. 2008; 73:16–20.(33). Janbaz K. H., Gilani A. H.J. Ethnopharmacol. 1995; 47:43–47.(34). Benli M., Kaya I., Yigit N.Cell Biochem. Funct. 2007; 25:681–686.(35). Shahriyary L., Yazdanparast R.J. Ethnopharmacol. 2007; 114:194–198.(36). Aniya Y., Shimabukuro M., Shimoji M., Kohatsu M., Gyamfi M. A., Miyagi C., Kunii D., Takayama F., Egashira T.Biol. Pharm. Bull. 2000; 23:309–312.(37). Zhang Z., Guo S. S., Zhang W. J., Geng Z. F., Liang J. Y., Du S. S., Wang C. F., Deng Z. W.Ind. Crops Prod. 2017; 100:132–137.(38). Islam M. N., Choi R. J., Jung H. A., Oh S. H., Choi J. S.Arch. Pharm. Res. 2016; 39:340–349.(39). Tanaka K.Seikagaku. 1961; 33:399–409.(40). Masuda Y., Asada K., Satoh R., Takada K., Kitajima J.Phytomedicine. 2015; 22:545–552.(41). Jihed B., Bhouri W., Ben Sghaier M., Bouhlel I., Kriffi M., Skandrani I., Dijoux F. M., Ghedira K., Chekir-Ghedira L.Nutr. Cancer. 2012; 64:1095–1102.(42). Kiso Y., Ogasawara S., Hirota K., Watanabe N., Oshima Y., Konno C., Hikino H.Planta Med. 1984; 50:81–85.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chemical Constituents from the Aerial Parts of Artemisia capillaris Thunb. and Their Anti-allergic and Anti-inflammatory Effects
- Effect of Artemisia Capillaris Extract on the Growth of Food-Borne Pathogens
- What is In-Jin-Sook? Artemisia capillaris, Artemisia iwayomogi, and Artemisia annua
- Quercetin-3-O-β-D-Glucuronide Suppresses Lipopolysaccharide-Induced JNK and ERK Phosphorylation in LPS-Challenged RAW264.7 Cells
- Anti-Inflammatory Effects of Fermented Products with Avena sativa on RAW264.7 and HT-29 Cells via Inhibition of Inflammatory Mediators