Clin Exp Vaccine Res.
2016 Jul;5(2):169-174. 10.7774/cevr.2016.5.2.169.
A recombinant rabies virus (ERAGS) for use in a bait vaccine for swine
- Affiliations
-
- 1Viral Disease Division, Animal and Plant Quarantine Agency, MAFRA, Gimcheon, Korea. yangdk@korea.kr
- KMID: 2421564
- DOI: http://doi.org/10.7774/cevr.2016.5.2.169
Abstract
- PURPOSE
Rabies viruses (RABV) circulating worldwide in various carnivores occasionally cause fatal encephalitis in swine. In this study, the safety and immunogenicity of a recombinant rabies virus, the ERAGS strain constructed with a reverse genetics system, was evaluated in domestic pigs.
MATERIALS AND METHODS
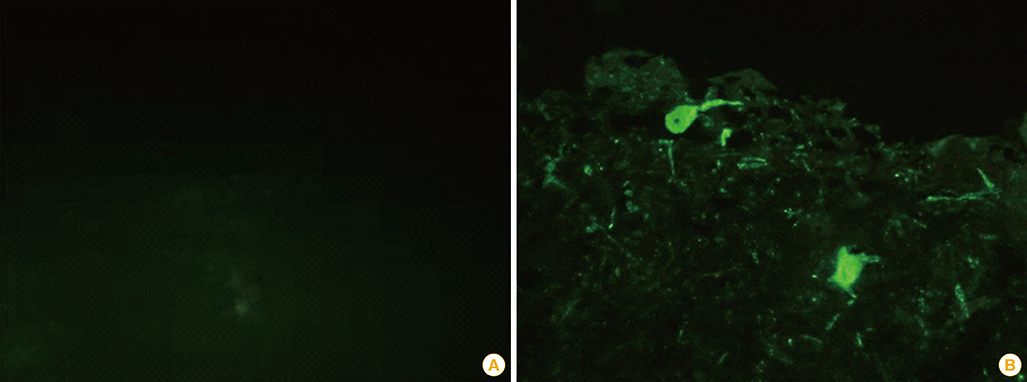
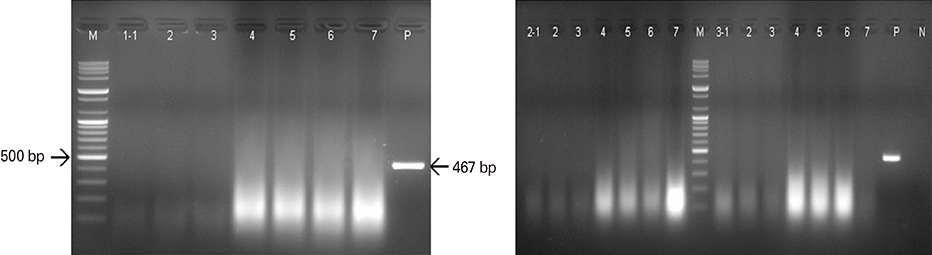
Growing pigs were administered 1 mL (108.0 FAID50/mL) of the ERAGS strain via intramuscular (IM) or oral routes and were observed for 4 weeks' post-inoculation. Three sows were also inoculated with 1 mL of the ERAGS strain via the IM route. The safety and immunogenicity in swine were evaluated using daily observation and a virus-neutralizing assay (VNA). Fluorescent antibody tests (FAT) for the RABV antigen and reverse transcriptase-polymerase chain reaction (RT-PCR) assays for the detection of the nucleocapsid (N) gene of RABV were conducted with brain tissues from the sows after necropsy.
RESULTS
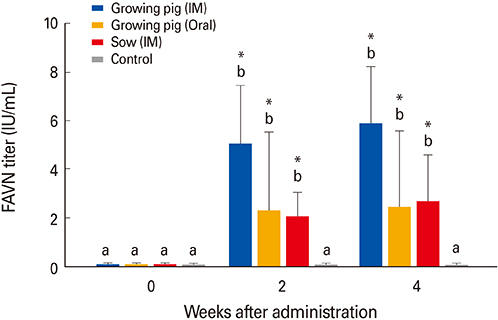
The growing pigs and sows administered the ERAGS strain did not exhibit any clinical sign of rabies during the test period test and did develop VNA titers. The growing pigs inoculated with the ERAGS strain via the IM route showed higher VNA titers than did those receiving oral administration. FAT and RT-PCR assays were unable to detect RABV in several tissues, including brain samples from the sows.
CONCLUSION
Our results suggest that the ERAGS strain was safe in growing pigs and sows and induced moderate VNA titers in pigs.
MeSH Terms
Figure
Reference
-
1. World Health Organization. WHO Expert Consultation on Rabies. World Health Organ Tech Rep Ser. 2005; 931:1–88.2. Yang DK, Park YN, Hong GS, et al. Molecular characterization of Korean rabies virus isolates. J Vet Sci. 2011; 12:57–63.
Article3. Baer GM, Olson HR. Recovery of pigs from rabies. J Am Vet Med Assoc. 1972; 160:1127–1128.4. Jiang Y, Yu X, Wang L, et al. An outbreak of pig rabies in Hunan province, China. Epidemiol Infect. 2008; 136:504–508.
Article5. Luo Y, Zhang Y, Liu X, et al. Complete genome sequence of a highly virulent rabies virus isolated from a rabid pig in south China. J Virol. 2012; 86:12454–12455.
Article6. DuVernoy TS, Mitchell KC, Myers RA, Walinski LW, Tinsley MO. The first laboratory-confirmed rabid pig in Maryland, 2003. Zoonoses Public Health. 2008; 55:431–435.
Article7. de Macedo Pessoa CR, Cristiny Rodrigues Silva ML, de Barros Gomes AA, et al. Paralytic rabies in Swine. Braz J Microbiol. 2011; 42:298–302.
Article8. Kim YR. Prophylaxis of human hydrophobia in South Korea. Infect Chemother. 2014; 46:143–148.
Article9. Yang DK, Nakagawa K, Ito N, et al. A single immunization with recombinant rabies virus (ERAG3G) confers complete protection against rabies in mice. Clin Exp Vaccine Res. 2014; 3:176–184.
Article10. Yang DK, Kim HH, Jo HY, Kim HW, Choi SS, Cho IS. Safety and immunogenicity of a recombinant rabies virus strain (ERAG3G) in Korean raccoon dogs. J Bacteriol Virol. 2015; 45:250–255.
Article11. Faber M, Dietzschold B, Li J. Immunogenicity and safety of recombinant rabies viruses used for oral vaccination of stray dogs and wildlife. Zoonoses Public Health. 2009; 56:262–269.
Article12. World Organization for Animal Health. Manual of diagnostic tests and vaccines for terrestrial animals. 6th ed. Paris: World Organization for Animal Health;2012. p. 263–282.13. Brochier B, Blancou J, Thomas I, et al. Use of recombinant vaccinia-rabies glycoprotein virus for oral vaccination of wildlife against rabies: innocuity to several non-target bait consuming species. J Wildl Dis. 1989; 25:540–547.
Article14. Brown LJ, Rosatte RC, Fehlner-Gardiner C, et al. Oral vaccination and protection of striped skunks (Mephitis mephitis) against rabies using ONRAB(R). Vaccine. 2014; 32:3675–3679.
Article15. Cliquet F, Aubert M, Sagne L. Development of a fluorescent antibody virus neutralisation test (FAVN test) for the quantitation of rabies-neutralising antibody. J Immunol Methods. 1998; 212:79–87.
Article16. Wright E, Temperton NJ, Marston DA, McElhinney LM, Fooks AR, Weiss RA. Investigating antibody neutralization of lyssaviruses using lentiviral pseudotypes: a cross-species comparison. J Gen Virol. 2008; 89(Pt 9):2204–2213.
Article17. Nandi S, Kumar M. Development in immunoprophylaxis against rabies for animals and humans. Avicenna J Med Biotechnol. 2010; 2:3–21.18. Joo YS, Lee JH, Lee KK, Bang HA, Lee WC. Retrospective study of extensive vaccination programs for canine rabies control and public health in Korea. Jpn J Infect Dis. 2011; 64:513–515.19. Yang DK, Kim HH, Lee KW, Song JY. The present and future of rabies vaccine in animals. Clin Exp Vaccine Res. 2013; 2:19–25.
Article20. Artois M, Guittre C, Thomas I, Leblois H, Brochier B, Barrat J. Potential pathogenicity for rodents of vaccines intended for oral vaccination against rabies: a comparison. Vaccine. 1992; 10:524–528.
Article21. Masson E, Cliquet F, Aubert M, et al. Safety study of the SAG2 rabies virus mutant in several non-target species with a view to its future use for the immunization of foxes in Europe. Vaccine. 1996; 14:1506–1510.
Article22. Jakubik J. Comparative susceptibility of rabbits, rats, mice and pigs to infection with Aujeszky diseases virus (ADV) in the development of an efficacy test for ADV vaccines. Zentralbl Veterinarmed B. 1977; 24:764–766.
Article23. Vengust G, Hostnik P, Cerovsek M, Cilensek P, Malovrh T. Presence of antibodies against rabies in wild boars. Acta Vet Hung. 2011; 59:149–154.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Establishment of multiplex RT-PCR for differentiation between rabies virus with and that without mutation at position 333 of glycoprotein
- Safety and immunogenicity of recombinant rabies virus (ERAGS) in mice and raccoon dogs
- Immunogenicity of an inactivated rabies vaccine for animals derived from the recombinant ERAGS strain
- The present and future of rabies vaccine in animals
- Generation of a recombinant rabies virus expressing green fluorescent protein for a virus neutralization antibody assay