J Breast Cancer.
2018 Sep;21(3):267-276. 10.4048/jbc.2018.21.e43.
Deficiency of Follistatin-Like Protein 1 Accelerates the Growth of Breast Cancer Cells at Lung Metastatic Sites
- Affiliations
-
- 1Department of Human Anatomy, School of Basic Medical Sciences, Capital Medical University, Beijing, China. gy1003@ccmu.edu.cn
- 2Department of Breast Surgery, Cancer Institute and Hospital, Chinese Academy of Medical Sciences, Beijing, China.
- 3Beijing Key Laboratory of Cancer Invasion and Metastasis Research, Beijing, China.
- 4Cancer Institute, Capital Medical University, Beijing, China.
- 5Department of Breast Surgery, Cancer Hospital of Huanxing Chaoyang District Beijing, Beijing, China.
- 6Department of Clinical Laboratory, Cancer Hospital of Huanxing Chaoyang District Beijing, Beijing, China.
- KMID: 2421367
- DOI: http://doi.org/10.4048/jbc.2018.21.e43
Abstract
- PURPOSE
Follistatin-like protein 1 (FSTL1) is a secreted glycoprotein that has been shown to play a role in various types of cancer. However, the clinical significance and function of FSTL1 in breast cancer have not been reported. We investigated the role of FSTL1 in breast cancer in this study.
METHODS
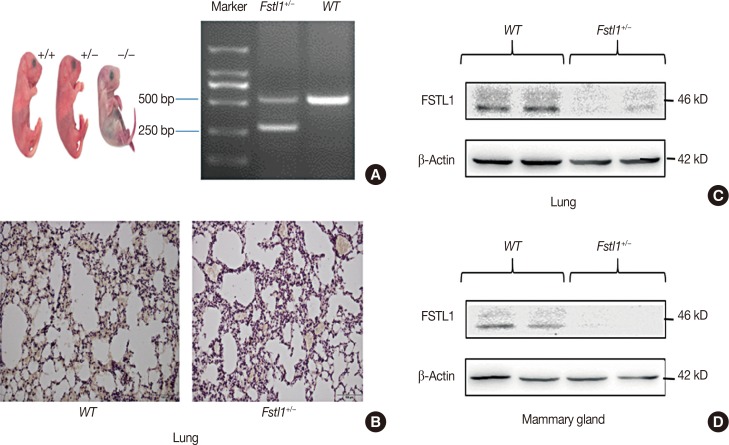
Enzyme-linked immunosorbent assays, western blot analysis, and reverse transcription polymerase chain reaction were used to monitor the expression of FSTL1 in breast cancer tissue and in serum samples from breast cancer patients. We employed a 4T1 breast cancer model and Fstl1(+/−) mice for in vivo studies. Hematoxylin and eosin staining, western blot analysis, and RNA sequencing were used to analyze the effect of FSTL1 on primary tumor growth and lung metastasis.
RESULTS
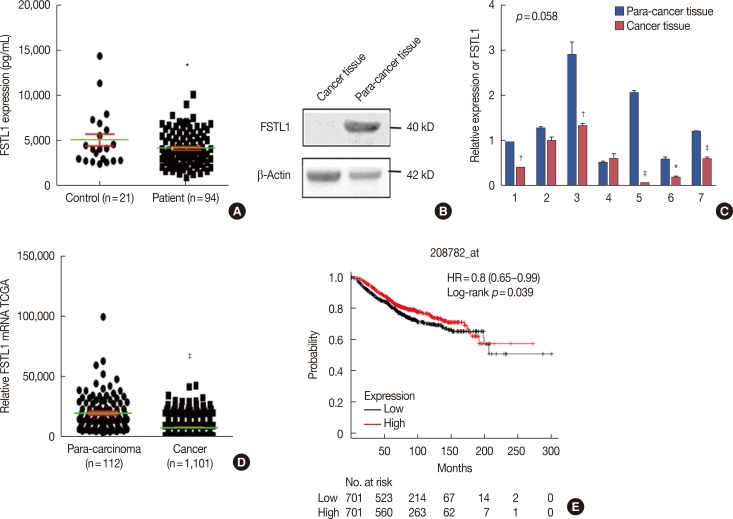
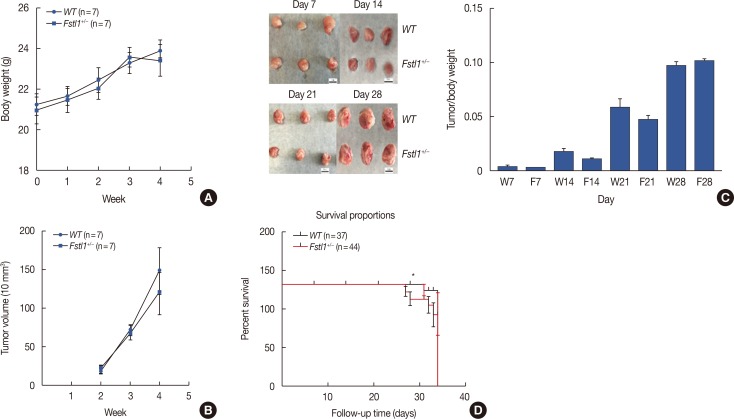
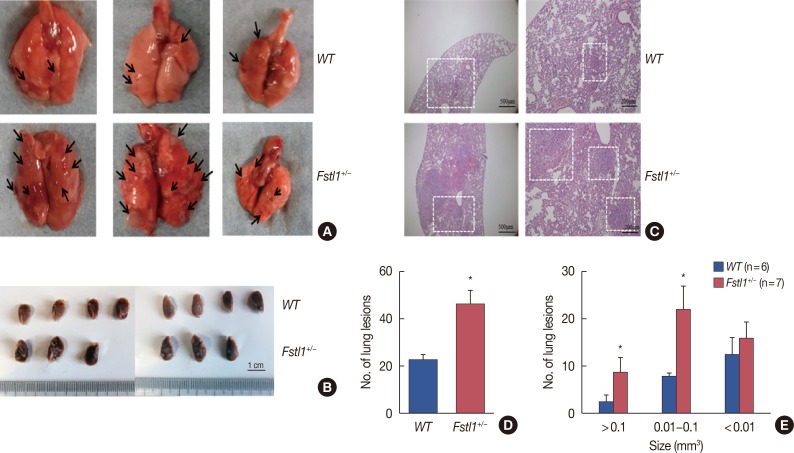
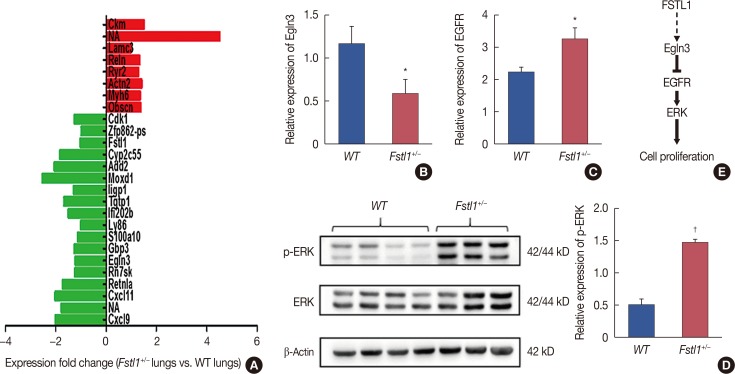
We demonstrated that the expression of FSTL1 is reduced in both the breast cancer tissue and the serum of breast cancer patients. We showed that reduced levels of FSTL1 in serum correlate with elevated expression of Ki-67 and epidermal growth factor receptor (EGFR) in cancer tissues. Moreover, lowered expression of FSTL1 was associated with decreased survival in breast cancer patients. Experiments on the Fstl1(+/−) mouse model established that FSTL1 deficiency had no effect on primary tumor growth, but increased the lung metastases of breast cancer cells, resulting in reduced survival of tumor-bearing mice. RNA sequencing found significantly reduced expression of Egln3 and increased expression of EGFR in Fstl1(+/−) mice. Thus, our results suggest that FSTL1 may affect the expression of EGFR through Egln3, inhibiting the proliferation of breast cancer cells at lung metastatic sites.
CONCLUSION
In conclusion, we suggest a suppressor role of FSTL1 in breast cancer lung metastasis. Furthermore, FSTL1 may represent a potential prognostic biomarker and a candidate therapeutic target in breast cancer patients.
MeSH Terms
-
Animals
Blotting, Western
Breast Neoplasms*
Breast*
Enzyme-Linked Immunosorbent Assay
Eosine Yellowish-(YS)
Follistatin-Related Proteins*
Genes, Tumor Suppressor
Glycoproteins
Hematoxylin
Humans
Lung*
Mice
Neoplasm Metastasis
Polymerase Chain Reaction
Receptor, Epidermal Growth Factor
Reverse Transcription
Sequence Analysis, RNA
Eosine Yellowish-(YS)
Follistatin-Related Proteins
Glycoproteins
Hematoxylin
Receptor, Epidermal Growth Factor
Figure
Reference
-
1. Li J, Zhang BN, Fan JH, Pang Y, Zhang P, Wang SL, et al. A nation-wide multicenter 10-year (1999–2008) retrospective clinical epidemiological study of female breast cancer in China. BMC Cancer. 2011; 11:364. PMID: 21859480.
Article2. Peddi PF, Ellis MJ, Ma C. Molecular basis of triple negative breast cancer and implications for therapy. Int J Breast Cancer. 2012; 2012:217185. PMID: 22295242.
Article3. Rakha EA, Elsheikh SE, Aleskandarany MA, Habashi HO, Green AR, Powe DG, et al. Triple-negative breast cancer: distinguishing between basal and nonbasal subtypes. Clin Cancer Res. 2009; 15:2302–2310. PMID: 19318481.
Article4. Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011; 121:2750–2767. PMID: 21633166.
Article5. Shibanuma M, Mashimo J, Mita A, Kuroki T, Nose K. Cloning from a mouse osteoblastic cell line of a set of transforming-growth-factor-beta 1-regulated genes, one of which seems to encode a follistatin-related polypeptide. Eur J Biochem. 1993; 217:13–19. PMID: 7901004.6. Geng Y, Dong Y, Yu M, Zhang L, Yan X, Sun J, et al. Follistatin-like 1 (Fstl1) is a bone morphogenetic protein (BMP) 4 signaling antagonist in controlling mouse lung development. Proc Natl Acad Sci U S A. 2011; 108:7058–7063. PMID: 21482757.
Article7. Li D, Wang Y, Xu N, Wei Q, Wu M, Li X, et al. Follistatin-like protein 1 is elevated in systemic autoimmune diseases and correlated with disease activity in patients with rheumatoid arthritis. Arthritis Res Ther. 2011; 13:R17. PMID: 21303509.
Article8. Oshima Y, Ouchi N, Sato K, Izumiya Y, Pimentel DR, Walsh K. Follistatin-like 1 is an Akt-regulated cardioprotective factor that is secreted by the heart. Circulation. 2008; 117:3099–3108. PMID: 18519848.
Article9. Reddy SP, Britto R, Vinnakota K, Aparna H, Sreepathi HK, Thota B, et al. Novel glioblastoma markers with diagnostic and prognostic value identified through transcriptome analysis. Clin Cancer Res. 2008; 14:2978–2987. PMID: 18483363.
Article10. Su S, Parris AB, Grossman G, Mohler JL, Wang Z, Wilson EM. Up-regulation of follistatin-like 1 by the androgen receptor and melanoma antigen-a11 in prostate cancer. Prostate. 2017; 77:505–516. PMID: 27976415.
Article11. Lau MC, Ng KY, Wong TL, Tong M, Lee TK, Ming XY, et al. FSTL1 promotes metastasis and chemoresistance in esophageal squamous cell carcinoma through NFκB-BMP signaling cross-talk. Cancer Res. 2017; 77:5886–5899. PMID: 28883005.
Article12. Zhou X, Xiao X, Huang T, Du C, Wang S, Mo Y, et al. Epigenetic inactivation of follistatin-like 1 mediates tumor immune evasion in nasopharyngeal carcinoma. Oncotarget. 2016; 7:16433–16444. PMID: 26918942.
Article13. Zhao W, Han HB, Zhang ZQ. Suppression of lung cancer cell invasion and metastasis by connexin43 involves the secretion of follistatin-like 1 mediated via histone acetylation. Int J Biochem Cell Biol. 2011; 43:1459–1468. PMID: 21718795.
Article14. Chan QK, Ngan HY, Ip PP, Liu VW, Xue WC, Cheung AN. Tumor suppressor effect of follistatin-like 1 in ovarian and endometrial carcinogenesis: a differential expression and functional analysis. Carcinogenesis. 2009; 30:114–121. PMID: 18796737.15. Hodgson G, Hager JH, Volik S, Hariono S, Wernick M, Moore D, et al. Genome scanning with array CGH delineates regional alterations in mouse islet carcinomas. Nat Genet. 2001; 29:459–464. PMID: 11694878.
Article16. Liu Y, Han X, Yu Y, Ding Y, Ni C, Liu W, et al. A genetic polymorphism affects the risk and prognosis of renal cell carcinoma: association with follistatin-like protein 1 expression. Sci Rep. 2016; 6:26689. PMID: 27225192.
Article17. Hatzimichael E, Dasoula A, Shah R, Syed N, Papoudou-Bai A, Coley HM, et al. The prolyl-hydroxylase EGLN3 and not EGLN1 is inactivated by methylation in plasma cell neoplasia. Eur J Haematol. 2010; 84:47–51. PMID: 19737309.
Article18. Garvalov BK, Foss F, Henze AT, Bethani I, Gräf-Höchst S, Singh D, et al. PHD3 regulates EGFR internalization and signalling in tumours. Nat Commun. 2014; 5:5577. PMID: 25420589.
Article19. Henze AT, Garvalov BK, Seidel S, Cuesta AM, Ritter M, Filatova A, et al. Loss of PHD3 allows tumours to overcome hypoxic growth inhibition and sustain proliferation through EGFR. Nat Commun. 2014; 5:5582. PMID: 25420773.
Article20. Meng X, Zheng R, Zhang Y, Qiao M, Liu L, Jing P, et al. An activated sympathetic nervous system affects white adipocyte differentiation and lipolysis in a rat model of Parkinson's disease. J Neurosci Res. 2015; 93:350–360. PMID: 25257318.
Article21. Su Y, Loos M, Giese N, Hines OJ, Diebold I, Görlach A, et al. PHD3 regulates differentiation, tumour growth and angiogenesis in pancreatic cancer. Br J Cancer. 2010; 103:1571–1579. PMID: 20978507.
Article22. Xue J, Li X, Jiao S, Wei Y, Wu G, Fang J. Prolyl hydroxylase-3 is down-regulated in colorectal cancer cells and inhibits IKKbeta independent of hydroxylase activity. Gastroenterology. 2010; 138:606–615. PMID: 19786027.23. Bae K, Park KE, Han J, Kim J, Kim K, Yoon KA. Mitotic cell death caused by follistatin-like 1 inhibition is associated with up-regulated Bim by inactivated Erk1/2 in human lung cancer cells. Oncotarget. 2016; 7:18076–18084. PMID: 26716515.
Article24. Shi DL, Shi GR, Xie J, Du XZ, Yang H. MicroRNA-27a inhibits cell migration and invasion of fibroblast-like synoviocytes by targeting follistatin-like protein 1 in rheumatoid arthritis. Mol Cells. 2016; 39:611–618. PMID: 27498552.
Article25. Tsou PS, Wren JD, Amin MA, Schiopu E, Fox DA, Khanna D, et al. Histone deacetylase 5 is overexpressed in scleroderma endothelial cells and impairs angiogenesis via repression of proangiogenic factors. Arthritis Rheumatol. 2016; 68:2975–2985. PMID: 27482699.
Article26. Witton CJ, Reeves JR, Going JJ, Cooke TG, Bartlett JM. Expression of the HER1-4 family of receptor tyrosine kinases in breast cancer. J Pathol. 2003; 200:290–297. PMID: 12845624.
Article27. An J, Wang L, Zhao Y, Hao Q, Zhang Y, Zhang J, et al. Effects of FSTL1 on cell proliferation in breast cancer cell line MDA MB 231 and its brain metastatic variant MDA MB 231 BR. Oncol Rep. 2017; 38:3001–3010. PMID: 29048681.28. Gao H, Chakraborty G, Zhang Z, Akalay I, Gadiya M, Gao Y, et al. Multi-organ site metastatic reactivation mediated by non-canonical discoidin domain receptor 1 signaling. Cell. 2016; 166:47–62. PMID: 27368100.
Article29. Miyabe M, Ohashi K, Shibata R, Uemura Y, Ogura Y, Yuasa D, et al. Muscle-derived follistatin-like 1 functions to reduce neointimal formation after vascular injury. Cardiovasc Res. 2014; 103:111–120. PMID: 24743592.
Article30. Trojan L, Schaaf A, Steidler A, Haak M, Thalmann G, Knoll T, et al. Identification of metastasis-associated genes in prostate cancer by genetic profiling of human prostate cancer cell lines. Anticancer Res. 2005; 25(1A):183–191. PMID: 15816537.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Influence of Activin and Follistatin on Expression of Insulin-like Growth Factor (IGF)-I, II, Insulin-like Growth Factor Binding Protein (IGFBP)-1, 2, and 3 mRNA in Cultured Mouse Granulosa Cells
- Advances in Clinically Relevant Metastatic Breast Cancer Models
- Clinicopathological Study of 191 Cases of Metastatic Skin Cancers from Solid Cancer Diagnosed at the Department of Dermatology in a Tertiary Referral Cancer Center
- Multiple Cutaneous Edema and Infiltration of Signet-ring Cells in the Lymphatics as an Initial Manifestation of Metastatic Gastric Adenocarcinoma
- Analysis of PIK3CA Mutation Concordance and Frequency in Primary and Different Distant Metastatic Sites in Breast Cancer