J Bacteriol Virol.
2018 Sep;48(3):73-80. 10.4167/jbv.2018.48.3.73.
Influence of Cell Surface Hydrophobicity on Adhesion and Biofilm Formation in Candida albicans and Several Bacterial Species
- Affiliations
-
- 1Department of Microbiology, Yonsei University Wonju College of Medicine, Wonju, Gangwon-do, Korea. leekh@yonsei.ac.kr
- KMID: 2421302
- DOI: http://doi.org/10.4167/jbv.2018.48.3.73
Abstract
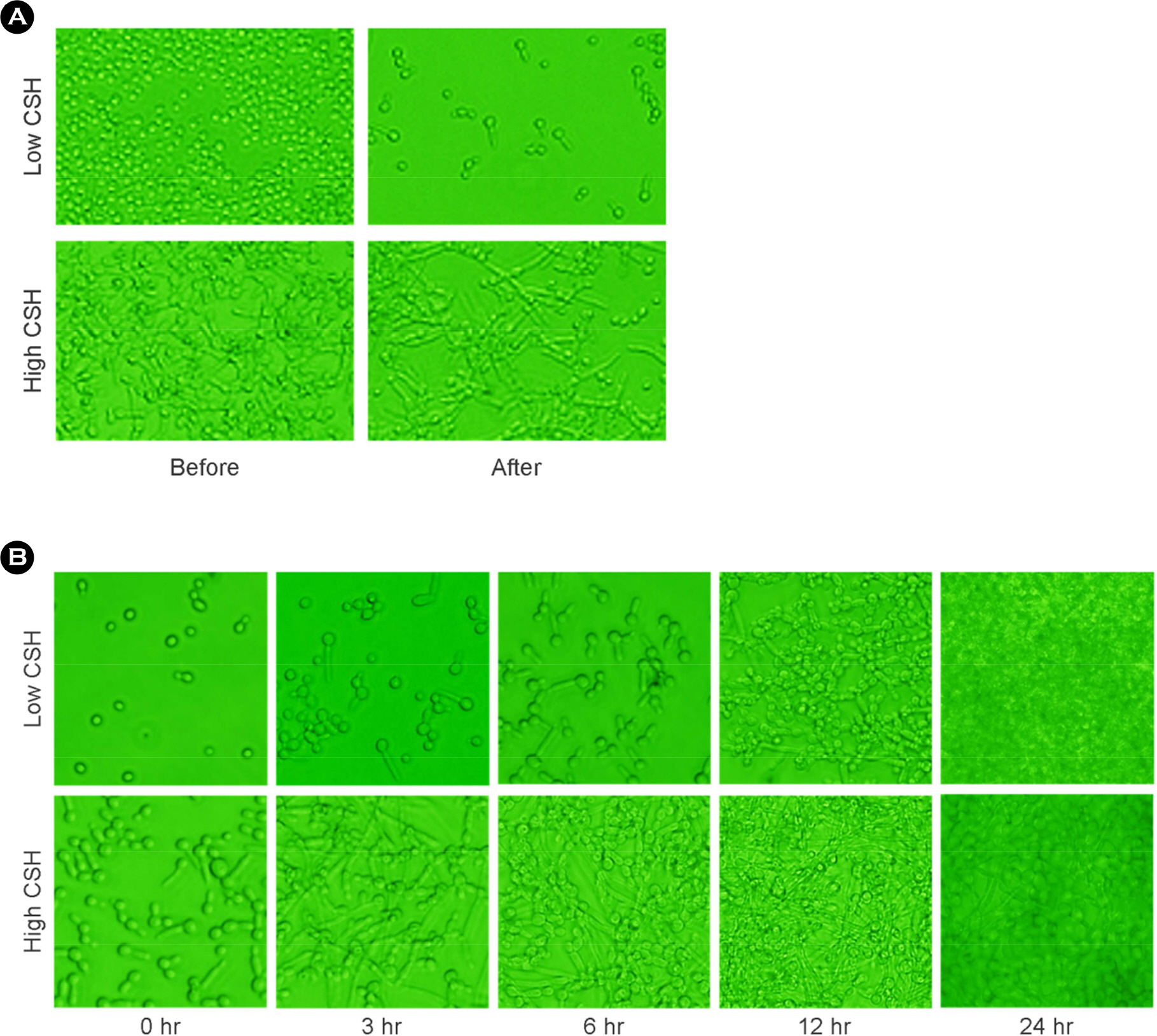
- The purpose of this study is to investigate the correlation of cell surface hydrophobicity (CSH) and biofilm formation or adhesion in Candida albicans (C. albicans) and several pathogenic bacteria. All of C. albicans (n=82) and 7 bacterial species (Escherichia coli, n=25; Klebsiella pneumoniae, n=33; Morganella morganii, n=21; Proteus mirabilis, n=33; Proteus vulgaris, n=12; Pseudomonas aeruginosa, n=31; Staphylococcus aureus, n=31) were isolated clinically. CSH was quantified with microbial adhesion to hydrocarbons. Biofilm formation was determined by tetrazolium salt reduction assay. Adhesion assay was performed by counting colonies after culture the microbes adhered to HeLa cells. Although high CSH-expressing bacterial species showed greater adherence to HeLa cells and larger amounts of biofilm formation on polystyrene, the significant relationships within same species were not shown. In C. albicans, however, strong positive correlations were observed between CSH and biofilm formation (r =0.708; p < 0.05) or cell adhesion (r =0.509; p < 0.05). These results suggest that hydrophobic force of bacteria may play a minor role in adhesion and biofilm formation, but CSH of C. albicans may be an important factor for adherence on surface and biofilm forming process.
MeSH Terms
Figure
Reference
-
1). O'Toole G, Kaplan HB, Kolter R. Biofilm formation as microbial development. Annu Rev Microbiol. 2000; 54:49–79.2). Chandra J, Kuhn DM, Mukherjee PK, Hoyer LL, McCormick T, Ghannoum MA. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J Bacteriol. 2001; 183:5385–94.3). Wey SB, Mori M, Pfaller MA, Woolson RF, Wenzel RP. Hospital-acquired candidemia. The attributable mortality and excess length of stay. Arch Intern Med. 1988; 148:2642–5.
Article4). Shirtliff ME, Peters BM, Jabra-Rizk MA. Cross-kingdom interactions: Candida albicans and bacteria. FEMS Microbiol Lett. 2009; 299:1–8.5). Samaranayake LP, MacFarlane TW, Williamson MI. Comparison of Sabouraud dextrose and Pagano-Levin agar media for detection and isolation of yeasts from oral samples. J Clin Microbiol. 1987; 25:162–4.
Article6). Webb BC, Thomas CJ, Willcox MD, Harty DW, Knox KW. Candida-associated denture stomatitis. Aetiology and management: a review. Part 2. Oral diseases caused by Candida species. Aust Dent J. 1998; 43:160–6.7). Lee KH, Park SJ, Choi S, Uh Y, Park JY, Han KH. The influence of urinary catheter materials on forming biofilms of microorganisms. J Bacteriol Virol. 2017; 47:32–40.
Article8). Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. Microbial biofilms. Annu Rev Microbiol. 1995; 49:711–45.
Article9). Borucki MK, Peppin JD, White D, Loge F, Call DR. Variation in biofilm formation among strains of Listeria monocytogenes. Appl Environ Microbiol. 2003; 69:7336–42.10). van Loosdrecht MC, Norde W, Zehnder AJ. Physical chemical description of bacterial adhesion. J Biomater Appl. 1990; 5:91–106.
Article11). Ambalam P, Kondepudi KK, Nilsson I, Wadström T, Ljungh A. Bile enhances cell surface hydrophobicity and biofilm formation of bifidobacteria. Appl Biochem Biotechnol. 2014; 172:1970–81.
Article12). Borecká-Melkusová S, Bujdáková H. Variation of cell surface hydrophobicity and biofilm formation among genotypes of Candida albicans and Candida dubliniensis under antifungal treatment. Can J Microbiol. 2008; 54:718–24.13). Khelissa SO, Jama C, Abdallah M, Boukherroub R, Faille C, Chihib NE. Effect of incubation duration, growth temperature, and abiotic surface type on cell surface properties, adhesion and pathogenicity of biofilm-detached Staphylococcus aureus cells. AMB Express. 2017; 7:216.
Article14). Ramage G, Vandewalle K, Wickes BL, López-Ribot JL. Characteristics of biofilm formation by Candida albicans. Rev Iberoam Micol. 2001; 18:163–70.15). Rosenberg M. Microbial adhesion to hydrocarbons: twenty-five years of doing MATH. FEMS Microbiol Lett. 2006; 262:129–34.
Article16). Pizarro-Cerdá J, Cossart P. Bacterial adhesion and entry into host cells. Cell. 2006; 24(124):715–27.
Article17). Hashemizadeh Z, Kalantar-Neyestanaki D, Mansouri S. Association between virulence profile, biofilm formation and phylogenetic groups of Escherichia coli causing urinary tract infection and the commensal gut microbiota: A comparative analysis. Microb Pathog. 2017; 110:540–5.18). Mirani ZA, Naz S, Khan F, Aziz M, Asadullah , Khan MN, et al. Antibacterial fatty acids destabilize hydrophobic and multicellular aggregates of biofilm in S. aureus. J Antibiot (Tokyo). 2017; 70:115–21.19). Ton-That H, Schneewind O. Assembly of pili in Gram-positive bacteria. Trends Microbiol. 2004; 12:228–34.
Article20). Poimenidou SV, Chrysadakou M, Tzakoniati A, Bikouli VC, Nychas GJ, Skandamis PN. Variability of Listeria monocytogenes strains in biofilm formation on stainless steel and polystyrene materials and resistance to peracetic acid and quaternary ammonium compounds. Int J Food Microbiol. 2016; 237:164–71.21). Schwarz-Linek U, Werner JM, Pickford AR, Gurusiddappa S, Kim JH, Pilka ES, et al. Pathogenic bacteria attach to human fibronectin through a tandem beta-zipper. Nature. 2003; 423:177–81.22). Linder M. Hydrophobins: Proteins that self-assemble at interfaces. Curr Coll Inter Sci. 2009; 14:356–63.23). Kurakado S, Takatori K, Sugita T. Minocycline Inhibits Candida albicans Budded-to-Hyphal-Form Transition and Biofilm Formation. Jpn J Infect Dis. 2017; 70:490–4.24). Liu T, Xu C, Hong L, Garcia-Godoy F, Hottel T, Babu J, et al. Effects of trimethylsilane plasma coating on the hydrophobicity of denture base resin and adhesion of Candida albicans on resin surfaces. J Prosthet Dent. 2017; 118:765–70.25). Lee JA. Robbins N, Xie JL, Ketela T, Cowen LE. Functional genomic analysis of Candida albicans adherence reveals a key role for the Arp2/3 complex in cell wall remodelling and biofilm formation. PLoS Genet. 2016; 12:e1006452.26). Park SJ, Han KH, Park JY, Choi SJ, Lee KH. Influence of bacterial presence on biofilm formation of Candida albicans. Yonsei Med J. 2014; 55:449–58.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Influence of Bacterial Presence on Biofilm Formation of Candida albicans
- Candida albicans Biofilm Formation and Pathophysiology
- Biofilm-forming ability and adherence to poly-(methyl-methacrylate) acrylic resin materials of oral Candida albicans strains isolated from HIV positive subjects
- Proteus vulgaris and Proteus mirabilis Decrease Candida albicans Biofilm Formation by Suppressing Morphological Transition to Its Hyphal Form
- Identification of Early Colonizing Bacteria Involved in Formation of Dental Unit Waterlines Biofilm Using TA Cloning