Yonsei Med J.
2017 Nov;58(6):1135-1143. 10.3349/ymj.2017.58.6.1135.
Proteus vulgaris and Proteus mirabilis Decrease Candida albicans Biofilm Formation by Suppressing Morphological Transition to Its Hyphal Form
- Affiliations
-
- 1Department of Microbiology, Yonsei University Wonju College of Medicine, Wonju, Korea. joopark@yonsei.ac.kr
- KMID: 2418895
- DOI: http://doi.org/10.3349/ymj.2017.58.6.1135
Abstract
- PURPOSE
Candida albicans (C. albicans) and Proteus species are causative agents in a variety of opportunistic nosocomial infections, and their ability to form biofilms is known to be a virulence factor. In this study, the influence of co-cultivation with Proteus vulgaris (P. vulgaris) and Proteus mirabilis (P. mirabilis) on C. albicans biofilm formation and its underlying mechanisms were examined.
MATERIALS AND METHODS
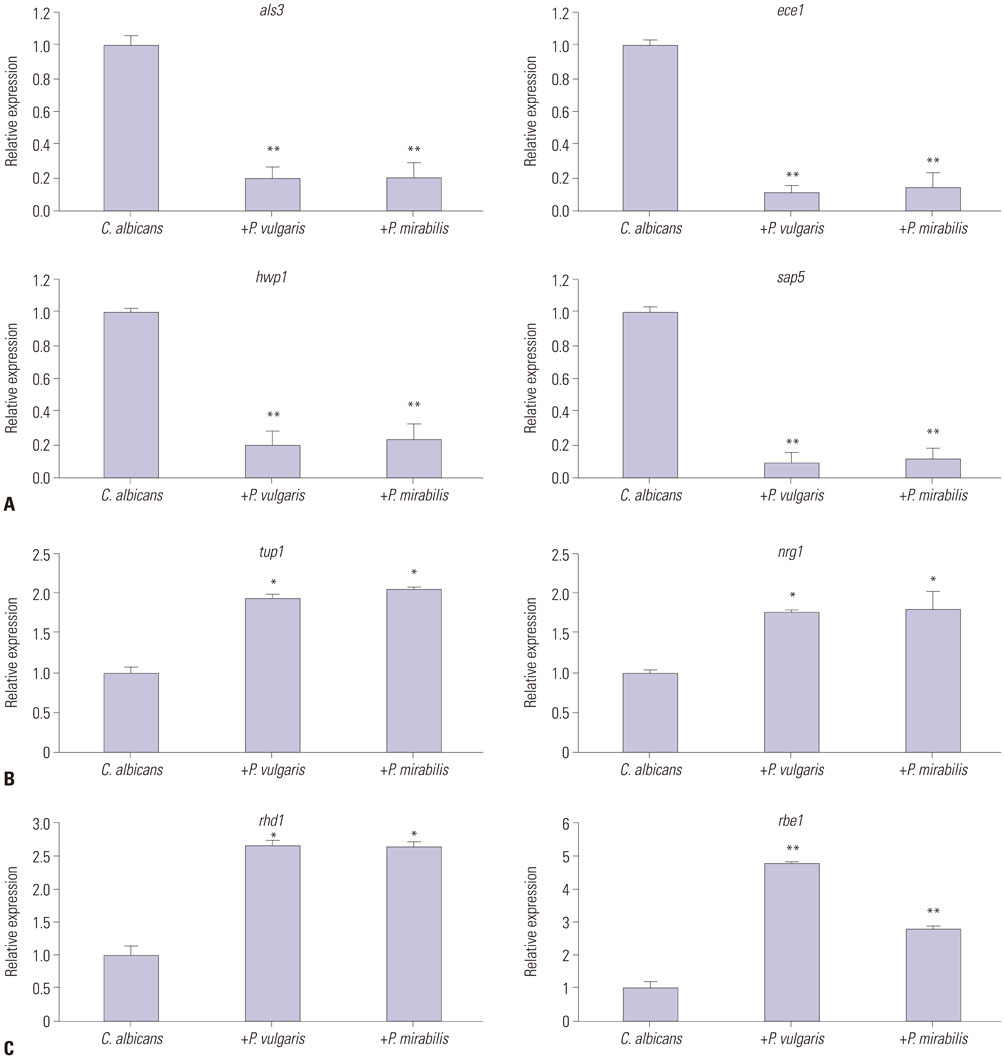
XTT reduction assays were adopted to measure biofilm formation, and viable colony counts were performed to quantify yeast growth. Real-time reverse transcriptase polymerase chain reaction was used to evaluate the expression of yeast-specific genes (rhd1 and rbe1), filament formation inhibiting genes (tup1 and nrg1), and hyphae-related genes (als3, ece1, hwp1, and sap5).
RESULTS
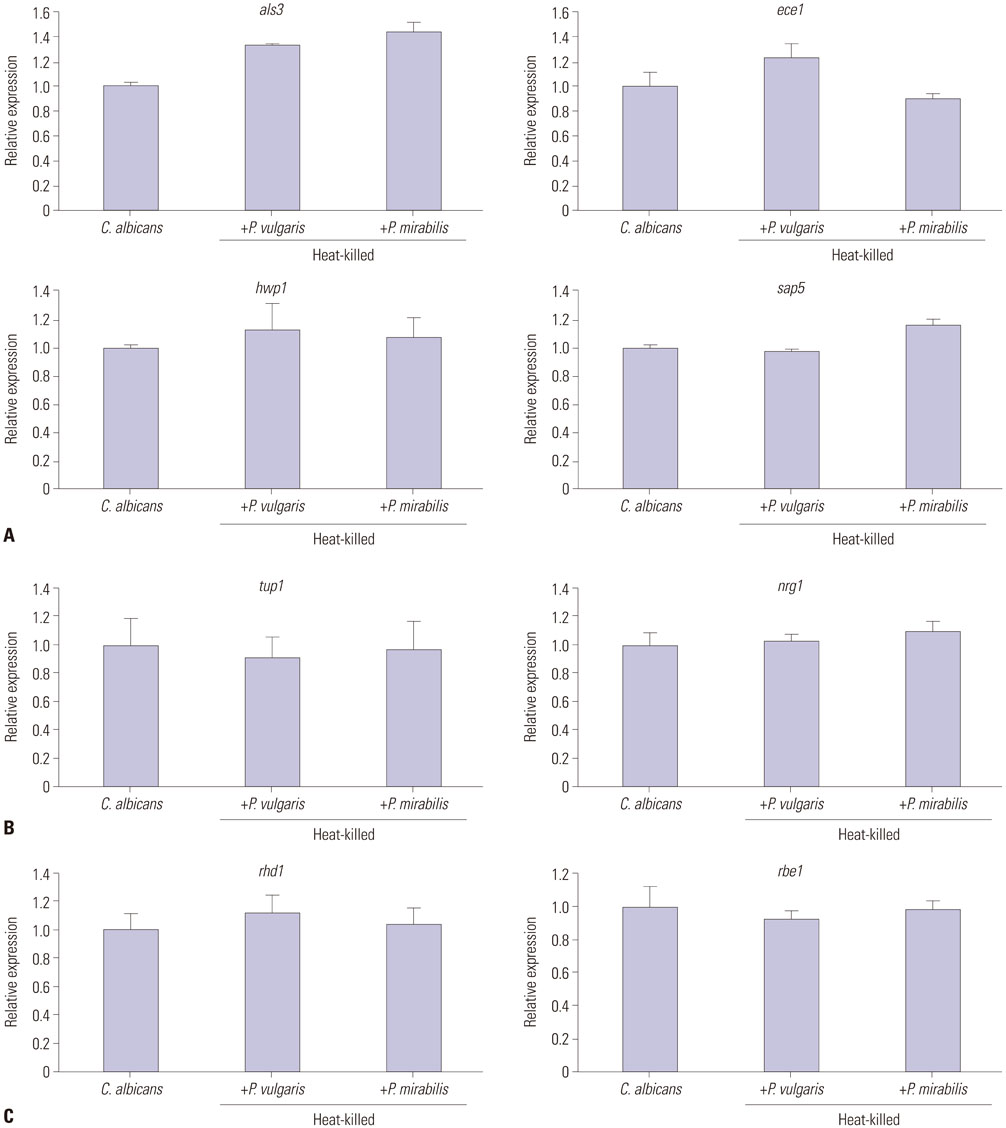
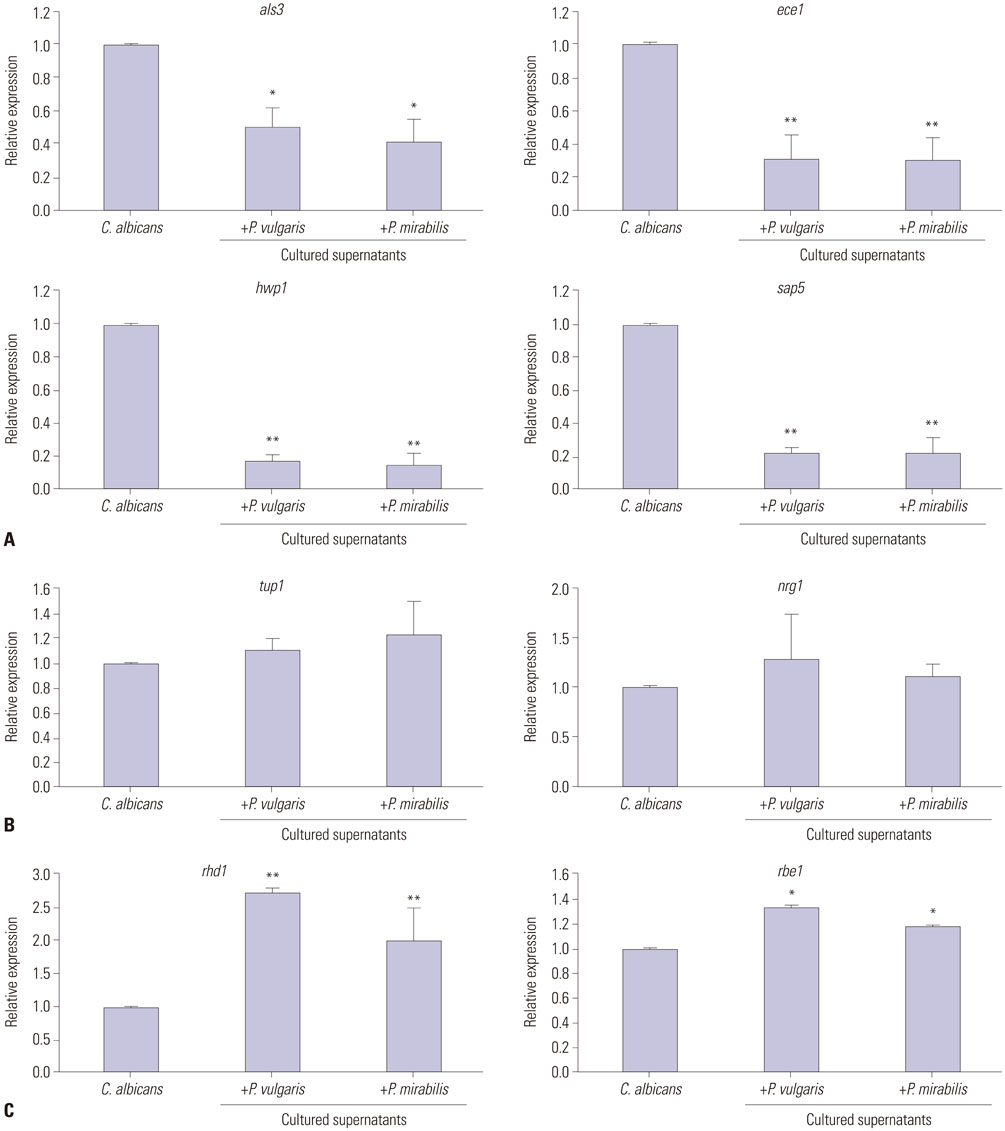
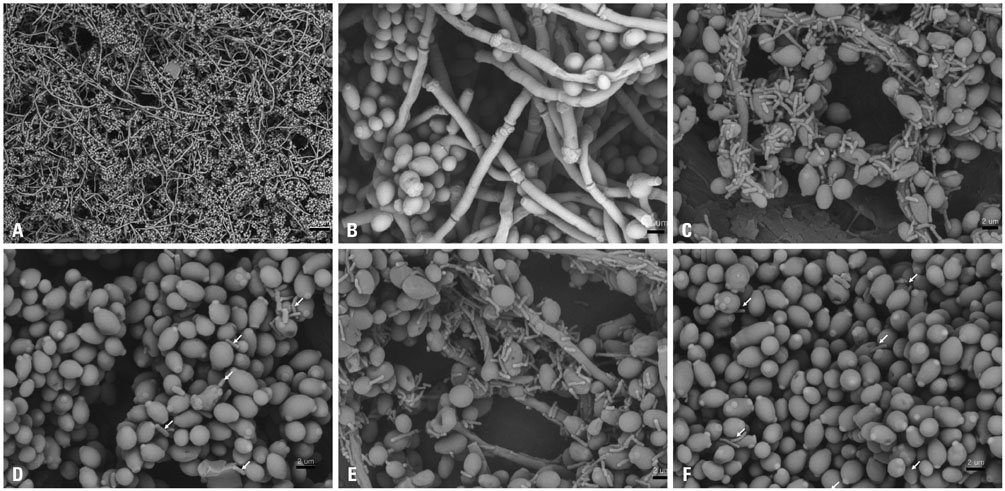
Candida biofilm formation was markedly inhibited by treatment with either living or heat-killed P. vulgaris and P. mirabilis. Proteus-cultured supernatant also inhibited Candida biofilm formation. Likewise, treatment with live P. vulgaris or P. mirabilis or with Proteus-cultured supernatant decreased expression of hyphae-related C. albicans genes, while the expression of yeast-specific genes and the filament formation inhibiting genes of C. albicans were increased. Heat-killed P. vulgaris and P. mirabilis treatment, however, did not affect the expression of C. albicans morphology-related genes.
CONCLUSION
These results suggest that secretory products from P. vulgaris and P. mirabilis regulate the expression of genes related to morphologic changes in C. albicans such that transition from the yeast form to the hyphal form can be inhibited.
Keyword
MeSH Terms
Figure
Reference
-
1. Potera C. Forging a link between biofilms and disease. Science. 1999; 283:1837–1839.
Article2. Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002; 15:167–193.
Article3. Fuqua C, Winans SC, Greenberg EP. Census and consensus in bacterial ecosystems: the LuxR-LuxI family of quorum-sensing transcriptional regulators. Annu Rev Microbiol. 1996; 50:727–751.
Article4. Ghannoum MA. Potential role of phospholipases in virulence and fungal pathogenesis. Clin Microbiol Rev. 2000; 13:122–143.
Article5. Sugita T, Kurosaka S, Yajitate M, Sato H, Nishikawa A. Extracellular proteinase and phospholipase activity of three genotypic strains of a human pathogenic yeast, Candida albicans. Microbiol Immunol. 2002; 46:881–883.
Article6. Hornby JM, Jensen EC, Lisec AD, Tasto JJ, Jahnke B, Shoemaker R, et al. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl Environ Microbiol. 2001; 67:2982–2992.
Article7. Douglas LJ. Candida biofilms and their role in infection. Trends Microbiol. 2003; 11:30–36.
Article8. Budtz-Jørgensen E, Mojon P, Rentsch A, Deslauriers N. Effects of an oral health program on the occurrence of oral candidosis in a long-term care facility. Community Dent Oral Epidemiol. 2000; 28:141–149.
Article9. Staab JF, Bradway SD, Fidel PL, Sundstrom P. Adhesive and mammalian transglutaminase substrate properties of Candida albicans Hwp1. Science. 1999; 283:1535–1538.
Article10. Hoyer LL. The ALS gene family of Candida albicans. Trends Microbiol. 2001; 9:176–180.
Article11. Liu H. Transcriptional control of dimorphism in Candida albicans. Curr Opin Microbiol. 2001; 4:728–735.
Article12. Granger BL. Insight into the antiadhesive effect of yeast wall protein 1 of Candida albicans. Eukaryot Cell. 2012; 11:795–805.
Article13. Sohn K, Urban C, Brunner H, Rupp S. EFG1 is a major regulator of cell wall dynamics in Candida albicans as revealed by DNA microarrays. Mol Microbiol. 2003; 47:89–102.
Article14. Znaidi S, Nesseir A, Chauvel M, Rossignol T, d'Enfert C. A comprehensive functional portrait of two heat shock factor-type transcriptional regulators involved in Candida albicans morphogenesis and virulence. PLoS Pathog. 2013; 9:e1003519.
Article15. Braun BR, Head WS, Wang MX, Johnson AD. Identification and characterization of TUP1-regulated genes in Candida albicans. Genetics. 2000; 156:31–44.
Article16. Rózalski A, Sidorczyk Z, Kotełko K. Potential virulence factors of Proteus bacilli. Microbiol Mol Biol Rev. 1997; 61:65–89.
Article17. Jacobsen SM, Stickler DJ, Mobley HL, Shirtliff ME. Complicated catheter-associated urinary tract infections due to Escherichia coli and Proteus mirabilis. Clin Microbiol Rev. 2008; 21:26–59.
Article18. Jacobsen SM, Shirtliff ME. Proteus mirabilis biofilms and catheter-associated urinary tract infections. Virulence. 2011; 2:460–465.
Article19. Thein ZM, Seneviratne CJ, Samaranayake YH, Samaranayake LP. Community lifestyle of Candida in mixed biofilms: a mini review. Mycoses. 2009; 52:467–475.20. Adam B, Baillie GS, Douglas LJ. Mixed species biofilms of Candida albicans and Staphylococcus epidermidis. J Med Microbiol. 2002; 51:344–349.
Article21. Costerton JW, Geesey GG, Cheng KJ. How bacteria stick. Sci Am. 1978; 238:86–95.
Article22. Park SJ, Han KH, Park JY, Choi SJ, Lee KH. Influence of bacterial presence on biofilm formation of Candida albicans. Yonsei Med J. 2014; 55:449–458.
Article23. Ramage G, Vandewalle K, Wickes BL, López-Ribot JL. Characteristics of biofilm formation by Candida albicans. Rev Iberoam Micol. 2001; 18:163–170.24. Fux CA, Costerton JW, Stewart PS, Stoodley P. Survival strategies of infectious biofilms. Trends Microbiol. 2005; 13:34–40.
Article25. Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol. 2004; 2:95–108.
Article26. Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. Microbial biofilms. Annu Rev Microbiol. 1995; 49:711–745.
Article27. Hansen SK, Rainey PB, Haagensen JA, Molin S. Evolution of species interactions in a biofilm community. Nature. 2007; 445:533–536.
Article28. Nett J, Andes D. Candida albicans biofilm development, modeling a host-pathogen interaction. Curr Opin Microbiol. 2006; 9:340–345.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Influence of Cell Surface Hydrophobicity on Adhesion and Biofilm Formation in Candida albicans and Several Bacterial Species
- A Rare Case of Ecthyma Gangrenosum Caused by Proteus vulgaris and Candida albicans in a Patient with Castleman Disease
- Influence of Bacterial Presence on Biofilm Formation of Candida albicans
- Candida albicans Biofilm Formation and Pathophysiology
- Antifungal effect of electrolyzed hydrogen water on Candida albicans biofilm