Yonsei Med J.
2018 Oct;59(8):1004-1007. 10.3349/ymj.2018.59.8.1004.
Bronchiectasis and Recurrent Respiratory Infections with a De Novo STAT1 Gain-of-Function Variant: First Case in Korea
- Affiliations
-
- 1Department of Laboratory Medicine and Genetics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 2Division of Pulmonary and Critical Care Medicine, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. wjkoh@skku.edu
- 3Division of Pediatric Infectious Diseases and Immunodeficiency, Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 4Center for Clinical Medicine, Samsung Biomedical Research Institute, Samsung Medical Center, Seoul, Korea.
- 5Green Cross Genome, Yongin, Korea. changski.md@gmail.com
- KMID: 2419736
- DOI: http://doi.org/10.3349/ymj.2018.59.8.1004
Abstract
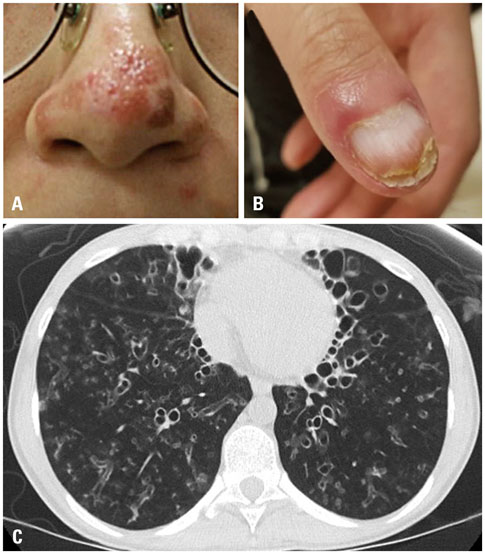
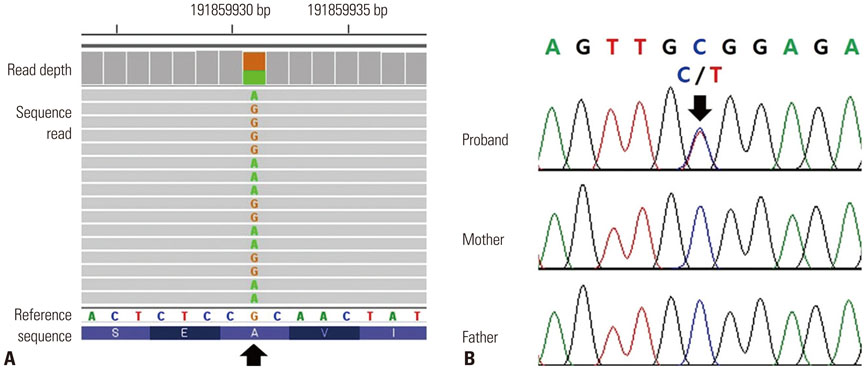
- Bronchiectasis is a chronic disease characterized by airway infection and inflammation, leading to permanent dilation of the bronchi. Evaluation of underlying etiology is important in managing young bronchiectasis patients with recurrent infections caused by unusual pathogens. The signal transducer and activator of transcription 1 (STAT1) protein plays a key role in STAT signaling and immune system regulation. Heterozygotes for gain-of-function (GOF) alleles of the STAT1 gene usually display autosomal dominant chronic mucocutaneous candidiasis (CMC) and a wide range of clinical features, such as bronchiectasis. Here, we report on a patient with CMC and bronchiectasis with various types of infections who carried a pathogenic variant of the STAT1 gene. The 24-year-old female presented with recurrent respiratory bacterial and nontuberculous mycobacterial infections complicated by severe bronchiectasis and CMC. Whole-exome sequencing revealed a c.800C>T (p.Ala267Val) heterozygous mutation in the STAT1 gene. Further analysis by Sanger sequencing of STAT1 from the patient and her parents revealed the patient had a de novo occurrence of the variant. This is the first report of a Korean patient with a GOF pathogenic variant in STAT1. Physicians should be aware of the existence of this variant as a genetic factor associated with CMC and bronchiectasis complicated by recurrent infection.
Keyword
MeSH Terms
Figure
Reference
-
1. Polverino E, Goeminne PC, McDonnell MJ, Aliberti S, Marshall SE, Loebinger MR, et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J. 2017; 50:1700629.
Article2. Chalmers JD, Aliberti S, Blasi F. Management of bronchiectasis in adults. Eur Respir J. 2015; 45:1446–1462.
Article3. Pasteur MC, Bilton D, Hill AT. British Thoracic Society Bronchiectasis non-CF Guideline Group. British Thoracic Society guideline for non-CF bronchiectasis. Thorax. 2010; 65:Suppl 1. i1–i58.
Article4. Araújo D, Shteinberg M, Aliberti S, Goeminne PC, Hill AT, Fardon T, et al. Standardised classification of the aetiology of bronchiectasis using an objective algorithm. Eur Respir J. 2017; 50:1701289.
Article5. Chapgier A, Boisson-Dupuis S, Jouanguy E, Vogt G, Feinberg J, Prochnicka-Chalufour A, et al. Novel STAT1 alleles in otherwise healthy patients with mycobacterial disease. PLoS Genet. 2006; 2:e131.6. van de Veerdonk FL, Plantinga TS, Hoischen A, Smeekens SP, Joosten LA, Gilissen C, et al. STAT1 mutations in autosomal dominant chronic mucocutaneous candidiasis. N Engl J Med. 2011; 365:54–61.
Article7. Boisson-Dupuis S, Kong XF, Okada S, Cypowyj S, Puel A, Abel L, et al. Inborn errors of human STAT1: allelic heterogeneity governs the diversity of immunological and infectious phenotypes. Curr Opin Immunol. 2012; 24:364–378.
Article8. Dupuis S, Dargemont C, Fieschi C, Thomassin N, Rosenzweig S, Harris J, et al. Impairment of mycobacterial but not viral immunity by a germline human STAT1 mutation. Science. 2001; 293:300–303.
Article9. Toubiana J, Okada S, Hiller J, Oleastro M, Lagos Gomez M, Aldave Becerra JC, et al. Heterozygous STAT1 gain-of-function mutations underlie an unexpectedly broad clinical phenotype. Blood. 2016; 127:3154–3164.
Article10. Depner M, Fuchs S, Raabe J, Frede N, Glocker C, Doffinger R, et al. The extended clinical phenotype of 26 patients with chronic mucocutaneous candidiasis due to gain-of-function mutations in STAT1. J Clin Immunol. 2016; 36:73–84.
Article11. Dotta L, Scomodon O, Padoan R, Timpano S, Plebani A, Soresina A, et al. Clinical and immunological data of nine patients with chronic mucocutaneous candidiasis disease. Data Brief. 2016; 7:311–315.
Article12. Dotta L, Scomodon O, Padoan R, Timpano S, Plebani A, Soresina A, et al. Clinical heterogeneity of dominant chronic mucocutaneous candidiasis disease: presenting as treatment-resistant candidiasis and chronic lung disease. Clin Immunol. 2016; 164:1–9.
Article13. Breuer O, Daum H, Cohen-Cymberknoh M, Unger S, Shoseyov D, Stepensky P, et al. Autosomal dominant gain of function STAT1 mutation and severe bronchiectasis. Respir Med. 2017; 126:39–45.
Article14. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015; 17:405–424.
Article15. Zheng J, van de Veerdonk FL, Crossland KL, Smeekens SP, Chan CM, Al Shehri T, et al. Gain-of-function STAT1 mutations impair STAT3 activity in patients with chronic mucocutaneous candidiasis (CMC). Eur J Immunol. 2015; 45:2834–2846.
Article16. Pedraza-Sánchez S, Lezana-Fernández JL, Gonzalez Y, Martínez-Robles L, Ventura-Ayala ML, Sadowinski-Pine S, et al. Disseminated tuberculosis and chronic mucocutaneous candidiasis in a patient with a gain-of-function mutation in signal transduction and activator of transcription 1. Front Immunol. 2017; 8:1651.
Article17. Koo S, Kejariwal D, Al-Shehri T, Dhar A, Lilic D. Oesophageal candidiasis and squamous cell cancer in patients with gain-of-function STAT1 gene mutation. United European Gastroenterol J. 2017; 5:625–631.
Article18. Sampaio EP, Hsu AP, Pechacek J, Bax HI, Dias DL, Paulson ML, et al. Signal transducer and activator of transcription 1 (STAT1) gain-of-function mutations and disseminated coccidioidomycosis and histoplasmosis. J Allergy Clin Immunol. 2013; 131:1624–1634.19. Bloomfield M, Kanderová V, Paračková Z, Vrabcová P, Svatoň M, Froňková E, et al. Utility of ruxolitinib in a child with chronic mucocutaneous candidiasis caused by a novel STAT1 gain-of-function mutation. J Clin Immunol. 2018; 38:589–601.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Secondary Immunodeficiency and Non-cystic Fibrosis Bronchiectasis
- A Clinical Study on Primary Ciliary Dyskinesia
- Early-Onset Parkinson’s Disease in a Patient With a De Novo Frameshift Variant of the ANKRD11 Gene and KBG Syndrome
- De novo HCN1 Mutation Identified by Next-Generation Sequencing in a Patient with Early Infantile Epileptic Encephalopathy: Case Report
- Identification of de novo BSCL2 Asn88Ser Variant with Atypical Presentation of Distal Hereditary Motor Neuropathy Type 5: Clinical Challenge in Diagnosis of Motor Neuron Diseases