J Nutr Health.
2018 Aug;51(4):275-286. 10.4163/jnh.2018.51.4.275.
Metabolites profiling and hypolipidemic/hypocholesterolemic effects of persimmon (Diosyros kaki Thumb.) by different processing procedures: in vitro and in vivo studies
- Affiliations
-
- 1Department of Nutritional Science and Food Management, Ewha Womans University, Seoul 03760, Korea. orank@ewha.ac.kr
- 2Ewha Graduate School of Converging Clinical&Public Health, Seoul 03760, Korea.
- 3Department of Food Science and Biotechnology, CHA University, Pocheon, Gyeonggi 11160, Korea.
- 4Department of Agrofood Resources, Rural Development Administration National Institute of Agricultural Sciences, Jeonju, Jeonbuk 55365, Korea.
- KMID: 2419498
- DOI: http://doi.org/10.4163/jnh.2018.51.4.275
Abstract
- PURPOSE
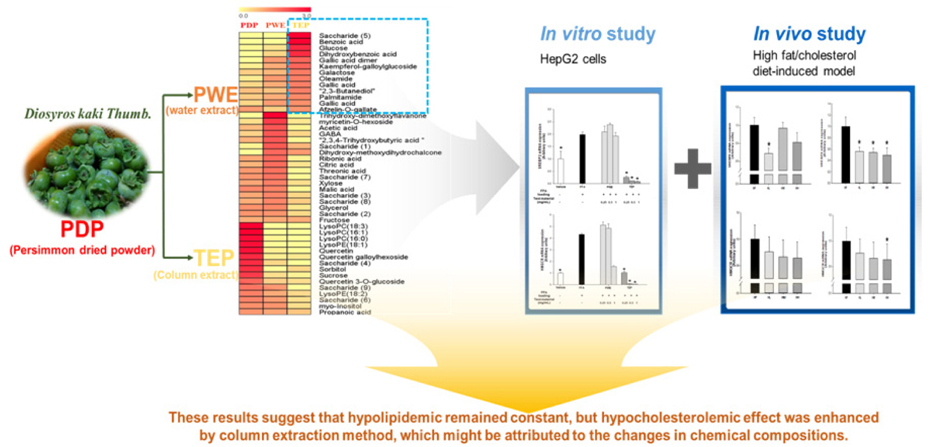
Our previous study demonstrated that persimmon (Diospyros kaki Thumb.) at different stages of ripening provided different protective effects against high-fat/cholesterol diet (HFD)-induced dyslipidemia in rats. In this study, we compared the metabolites profile and gene expressions related to triglyceride (TG)/cholesterol metabolism in vitro and in vivo after treating with persimmon water extracts (PWE) or tannin-enriched persimmon concentrate (TEP).
METHODS
Primary and secondary metabolites in test materials were determined by GC-TOF/MS, UHPLC-LTQ-ESI-IT-MS/MS, and UPLC-Q-TOF-MS. The expression of genes related to TG and cholesterol metabolism were determined by RT-PCR both in HepG2 cells stimulated by oleic acid/palmitic acid and in liver tissues obtained from Wistar rats fed with HFD and PWE at 0, 150, 300, and 600 mg/d (experiment I) or TEP at 0, 7, 14, and 28 mg/d (experiment II) by oral gavage for 9 weeks.
RESULTS
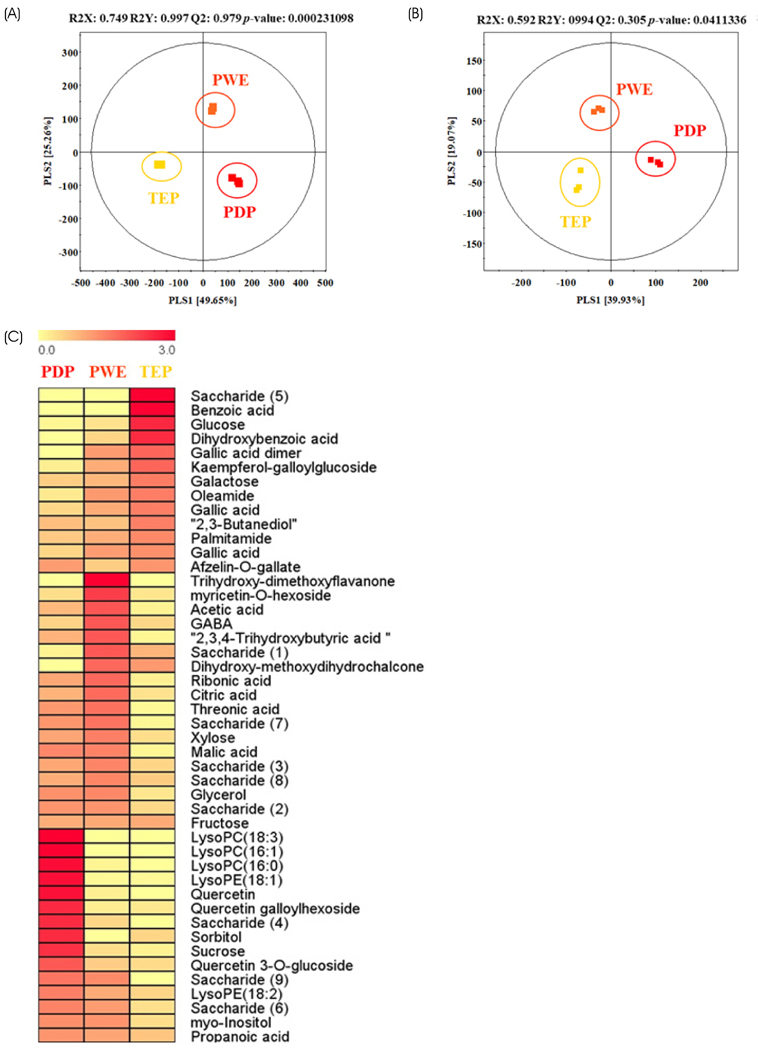
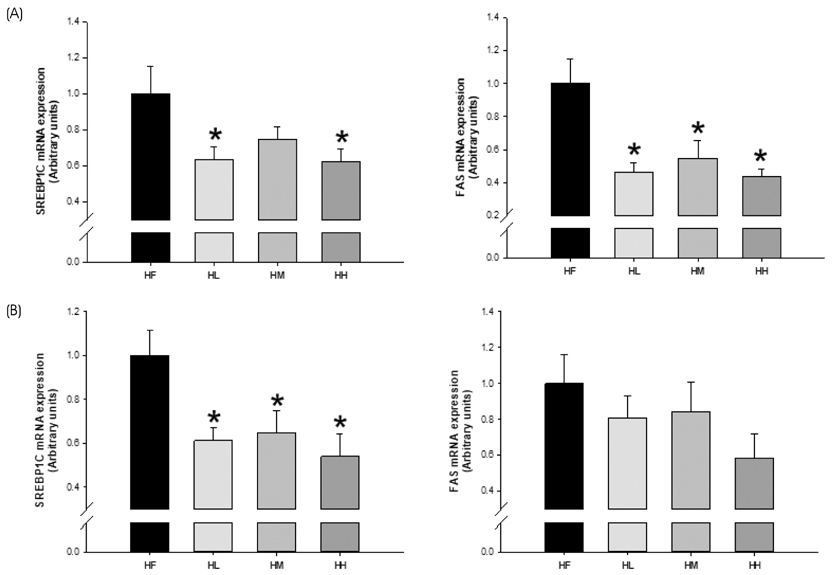
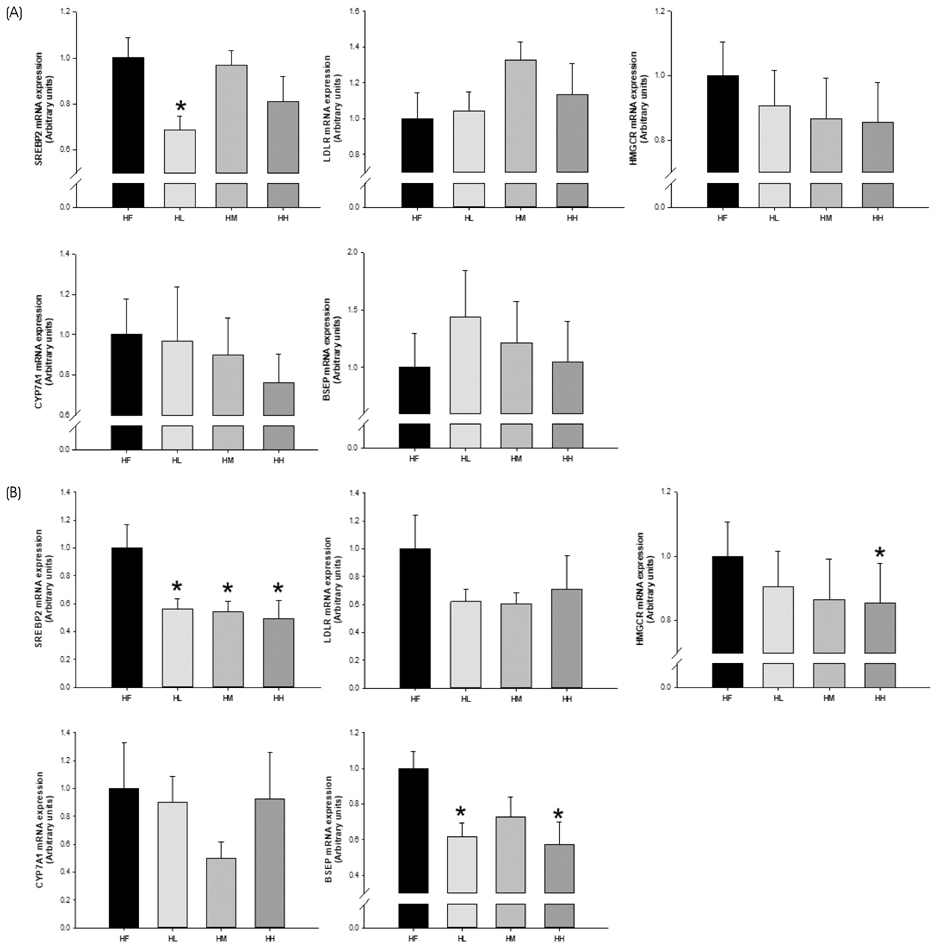
PLS-DA analysis and heatmap analysis demonstrated significantly differential profiling of metabolites of PWE and TEP according to processing of persimmon powder. In vitro, TEP showed similar hypolipidemic effects as PWE, but significantly enhanced hypocholesterolemic effects compared to PWE in sterol regulatory element-binding protein 2 (SREBP2), HMG-CoA reductase (HMGCR), proprotein convertase subtilisin/kexin type 9 (PCSK9), cholesterol 7α-hydroxylase (CYP7A1), and low density lipoprotein receptor (LDLR) gene expression. Consistently, TEP and PWE showed similar hypolipidemic capacity in vivo, but significantly enhanced hypocholesterolemic capacity in terms of SREBP2, HMGCR, and bile salt export pump (BSEP) gene expression.
CONCLUSION
These results suggest that column extraction after hot water extraction may be a good strategy to enhance tannins and long-chain fatty acid amides, which might cause stimulation of hypocholesterolemic actions through downregulation of cholesterol biosynthesis gene expression and upregulation of LDL receptor gene expression.
Keyword
MeSH Terms
-
Amides
Animals
Bile
Cholesterol
Diet
Diospyros*
Down-Regulation
Dyslipidemias
Gene Expression
Hep G2 Cells
In Vitro Techniques*
Liver
Metabolism
Oxidoreductases
Proprotein Convertases
Rats
Rats, Wistar
Receptors, LDL
Tannins
Triglycerides
Up-Regulation
Water
Amides
Cholesterol
Oxidoreductases
Proprotein Convertases
Receptors, LDL
Tannins
Water
Figure
Reference
-
1. Yokozawa T, Park CH, Noh JS, Roh SS. Role of oligomeric proanthocyanidins derived from an extract of persimmon fruits in the oxidative stress-related aging process. Molecules. 2014; 19(5):6707–6726.
Article2. Lee YA, Cho EJ, Tanaka T, Yokozawa T. Inhibitory activities of proanthocyanidins from persimmon against oxidative stress and digestive enzymes related to diabetes. J Nutr Sci Vitaminol (Tokyo). 2007; 53(3):287–292.
Article3. Zou B, Ge ZZ, Zhang Y, Du J, Xu Z, Li CM. Persimmon tannin accounts for hypolipidemic effects of persimmon through activating of AMPK and suppressing NF-κB activation and inflammatory responses in high-fat diet rats. Food Funct. 2014; 5(7):1536–1546.
Article4. Zou B, Li CM, Chen JY, Dong XQ, Zhang Y, Du J. High molecular weight persimmon tannin is a potent hypolipidemic in high-cholesterol diet fed rats. Food Res Int. 2012; 48(2):970–977.
Article5. Jang IC, Jo EK, Bae MS, Lee HJ, Jeon GI, Park E, Yuk HG, Ahn GH, Lee SC. Antioxidant and antigenotoxic activities of different parts of persimmon (Diospyros kaki cv. Fuyu) fruit. J Med Plant Res. 2010; 4(2):155–160.6. Matsumoto K, Yokoyama S, Gato N. Hypolipidemic effect of young persimmon fruit in C57BL/6.KOR-ApoEshl mice. Biosci Biotechnol Biochem. 2008; 72(10):2651–2659.7. Matsumoto K, Yokoyama S, Gato N. Bile acid-binding activity of young persimmon (Diospyros kaki) fruit and its hypolipidemic effect in mice. Phytother Res. 2010; 24(2):205–210.8. Ahn Y, Gebereamanuel MR, Oh EK, Kwon O. Inhibitory effects of persimmon (Diospyros kaki Thumb.) against diet-induced hypertriglyceridemia/hypercholesterolemia in rats. J Nutr Health. 2017; 50(3):225–235.9. Del Bubba M, Giordani E, Pippucci L, Cincinelli A, Checchini L, Galvan P. Changes in tannins, ascorbic acid and sugar content in astringent persimmons during on-tree growth and ripening and in response to different postharvest treatments. J Food Compos Anal. 2009; 22(7-8):668–677.
Article10. Association of Official Analytical Chemists. Official methods of analysis. 15th edition. Arlington (VA): Association of Official Analytical Chemists.11. Adams MA, Bobik A, Korner PI. Differential development of vascular and cardiac hypertrophy in genetic hypertension. Relation to sympathetic function. Hypertension. 1989; 14(2):191–202.
Article12. Karaman S, Toker ÖS, Yüksel F, Çam M, Kayacier A, Dogan M. Physicochemical, bioactive, and sensory properties of persimmon-based ice cream: technique for order preference by similarity to ideal solution to determine optimum concentration. J Dairy Sci. 2014; 97(1):97–110.
Article13. Dauchet L, Amouyel P, Hercberg S, Dallongeville J. Fruit and vegetable consumption and risk of coronary heart disease: a meta-analysis of cohort studies. J Nutr. 2006; 136(10):2588–2593.
Article14. Zhao D, Zhou C, Sheng Y, Liang G, Tao J. Molecular cloning and expression of phytoene synthase, lycopene beta-cyclase, and beta-carotene hydroxylase genes in persimmon (Diospyros kaki L.) fruits. Plant Mol Biol Report. 2011; 29(2):345–351.15. Santos AD, Fonseca FA, Dutra LM, Santos MF, Menezes LR, Campos FR, Nagata N, Ayub R, Barison A. 1H HR-MAS NMR-based metabolomics study of different persimmon cultivars (Diospyros kaki) during fruit development. Food Chem. 2018; 239:511–519.16. Bitou N, Ninomiya M, Tsujita T, Okuda H. Screening of lipase inhibitors from marine algae. Lipids. 1999; 34(5):441–445.
Article17. Latha RC, Daisy P. Insulin-secretagogue, antihyperlipidemic and other protective effects of gallic acid isolated from Terminalia bellerica Roxb. in streptozotocin-induced diabetic rats. Chem Biol Interact. 2011; 189(1-2):112–118.
Article18. Hsu CL, Yen GC. Effect of gallic acid on high fat diet-induced dyslipidaemia, hepatosteatosis and oxidative stress in rats. Br J Nutr. 2007; 98(4):727–735.
Article19. Ngamukote S, Mäkynen K, Thilawech T, Adisakwattana S. Cholesterol-lowering activity of the major polyphenols in grape seed. Molecules. 2011; 16(6):5054–5061.
Article20. Roth BD, Blankley CJ, Hoefle ML, Holmes A, Roark WH, Trivedi BK, Essenburg AD, Kieft KA, Krause BR, Stanfield RL. Inhibitors of acyl-CoA:cholesterol acyltransferase. 1. Identification and structure-activity relationships of a novel series of fatty acid anilide hypocholesterolemic agents. J Med Chem. 1992; 35(9):1609–1617.
Article21. Seo JB, Moon HM, Kim WS, Lee YS, Jeong HW, Yoo EJ, Ham J, Kang H, Park MG, Steffensen KR, Stulnig TM, Gustafsson JA, Park SD, Kim JB. Activated liver X receptors stimulate adipocyte differentiation through induction of peroxisome proliferator-activated receptor γ expression. Mol Cell Biol. 2004; 24(8):3430–3444.
Article22. Chen W, Yang CC, Sheu HM, Seltmann H, Zouboulis CC. Expression of peroxisome proliferator-activated receptor and CCAAT/enhancer binding protein transcription factors in cultured human sebocytes. J Invest Dermatol. 2003; 121(3):441–447.
Article23. Lee SM, Han HW, Kim Y. JNK-mediated SREBP-2 processing by genistein up-regulates LDLR expression in HepG2 cells. Nutr Food Sci. 2014; 4:308.24. Russell DW, Setchell KD. Bile acid biosynthesis. Biochemistry. 1992; 31(20):4737–4749.
Article25. Hashimoto K, Cohen RN, Yamada M, Markan KR, Monden T, Satoh T, Mori M, Wondisford FE. Cross-talk between thyroid hormone receptor and liver X receptor regulatory pathways is revealed in a thyroid hormone resistance mouse model. J Biol Chem. 2006; 281(1):295–302.
Article26. Cohen JC, Boerwinkle E, Mosley TH Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006; 354(12):1264–1272.27. Li Y, Xu S, Mihaylova MM, Zheng B, Hou X, Jiang B, Park O, Luo Z, Lefai E, Shyy JY, Gao B, Wierzbicki M, Verbeuren TJ, Shaw RJ, Cohen RA, Zang M. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011; 13(4):376–388.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pestalotiopsis kaki sp. nov., a Novel Species Isolated from Persimmon Tree (Diospyros kaki) Bark in Korea
- Inhibitory effects of persimmon (Diospyros kaki Thumb.) against diet-induced hypertriglyceridemia/hypercholesterolemia in rats
- Antioxidant and immune-enhancing effects of Fuyu persimmons and Hachiya persimmons
- Anticancer Effect of Persimmon Leaf Extracts on Korean Gastric Cancer Cell
- Occurrence of Sooty Blotch and Flyspeck Disease on Sweet Persimmon in Korea