Yonsei Med J.
2017 Jul;58(4):778-785. 10.3349/ymj.2017.58.4.778.
Comparison of Efficacy of Intravenous Peramivir and Oral Oseltamivir for the Treatment of Influenza: Systematic Review and Meta-Analysis
- Affiliations
-
- 1Department of Internal Medicine, Jeju National University Hospital, Jeju National University School of Medicine, Jeju, Korea.
- 2Division of Pulmonary and Critical Care Medicine, Seoul Metropolitan Government-Seoul National University Boramae Medical Center, Seoul, Korea.
- 3Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Kyung Hee University Hospital at Gangdong, Kyung Hee University School of Medicine, Seoul, Korea. yhkim2007@hotmail.co.kr
- KMID: 2419084
- DOI: http://doi.org/10.3349/ymj.2017.58.4.778
Abstract
- PURPOSE
Peramivir is the first intravenously administered neuramidase inhibitor for immediate delivery of an effective single-dose treatment in patients with influenza. However, limited data are available on intravenous (IV) peramivir treatment compared to oral oseltamivir for these patients.
MATERIALS AND METHODS
With a systematic review and meta-analysis, we compared the efficacy of IV peramivir with oral oseltamivir for treatment of patients with seasonal influenza. MEDLINE, EMBASE, and Cochrane Central Register were searched for relevant clinical trials.
RESULTS
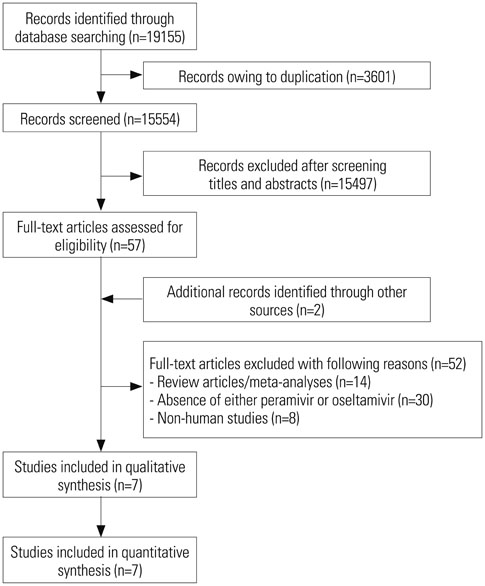
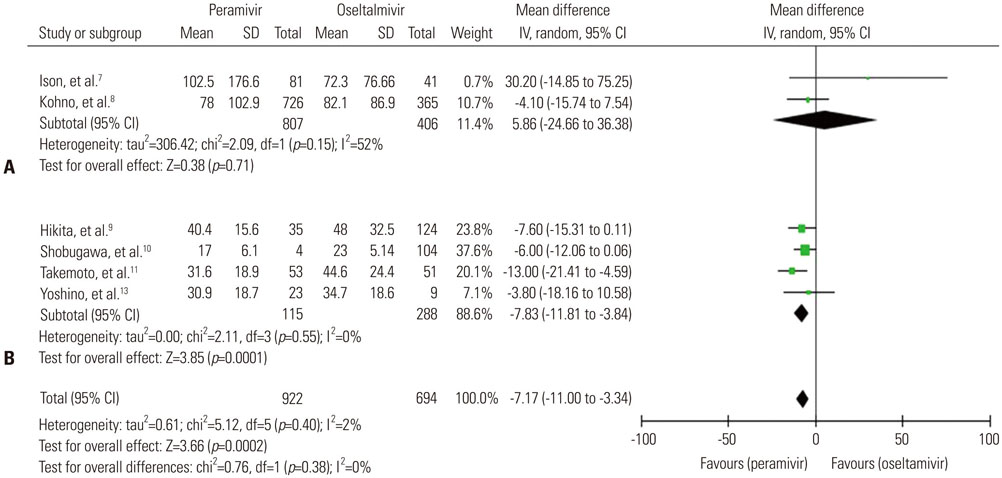
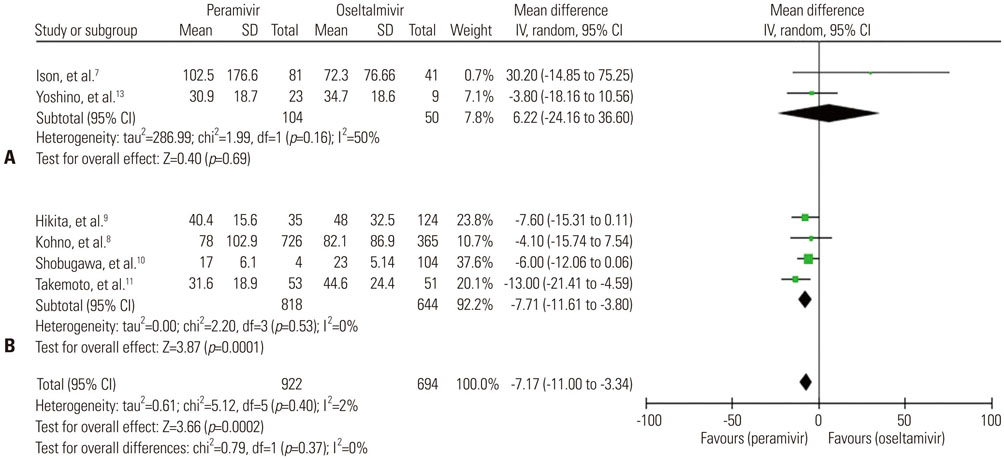
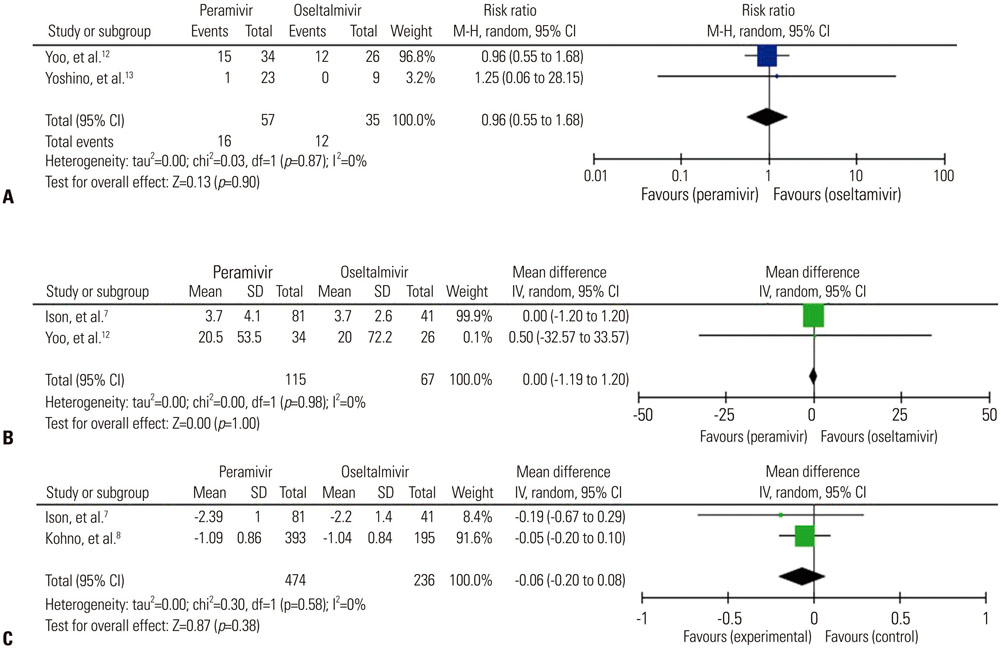
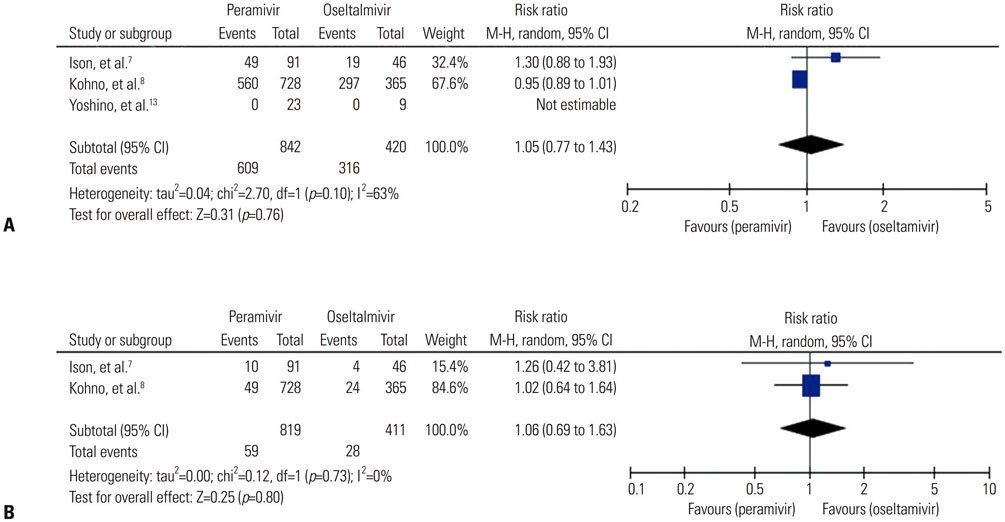
A total of seven trials [two randomized controlled trials (RCTs) and five non-randomized observational trials] involving 1676 patients were finally analyzed. The total number of peramivir- and oseltamivir-treated patients was 956 and 720, respectively. Overall, the time to alleviation of fever was lower in the peramivir-treated group compared with the oseltamivir-treated group [mean difference (MD), −7.17 hours; 95% confidence interval (CI) −11.00 to −3.34]. Especially, pooled analysis of observational studies (n=4) and studies of outpatients (n=4) demonstrated the superiority of the peramivir-treated group (MD, -7.83 hours; 95% CI −11.81 to −3.84 and MD, −7.71 hours; 95% CI −11.61 to −3.80, respectively). Mortality, length of hospital stay, change in virus titer 48 hours after admission, and the incidence of adverse events in these patients were not significantly different between the two groups.
CONCLUSION
IV peramivir therapy might reduce the time to alleviation of fever in comparison with oral oseltamivir therapy in patients with influenza; however, we could not draw clear conclusions from a meta-analysis because of the few RCTs available and methodological limitations.
Keyword
MeSH Terms
-
Administration, Intravenous
Administration, Oral
Antiviral Agents/administration & dosage/adverse effects/therapeutic use
Cyclopentanes/*administration & dosage/adverse effects/*therapeutic use
Guanidines/*administration & dosage/adverse effects/*therapeutic use
Humans
Influenza, Human/*drug therapy/virology
Odds Ratio
Oseltamivir/*administration & dosage/adverse effects/*therapeutic use
Publication Bias
Randomized Controlled Trials as Topic
Risk Factors
Treatment Outcome
Antiviral Agents
Cyclopentanes
Guanidines
Oseltamivir
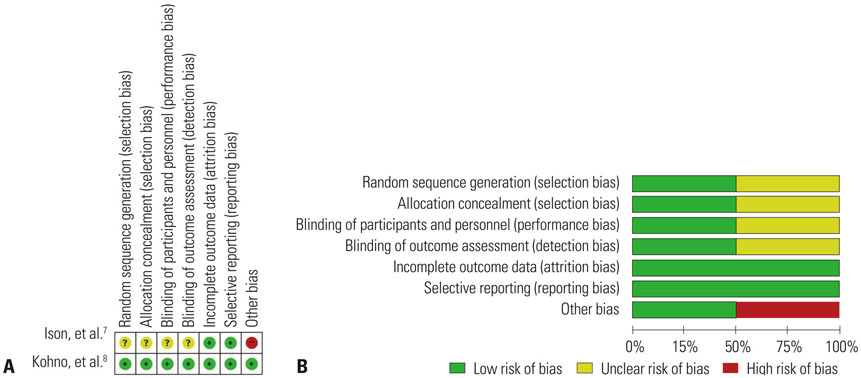
Figure
Cited by 1 articles
-
Safety and Effectiveness of Peramivir in Korean Adult Influenza Patients: Prospective Observational Study Based on Post-Marketing Surveillance Data
Won Suk Choi, Seong Yeol Ryu, Jacob Lee, Sang-Bum Hong, Joong Sik Eom, Jonghwan Shin, Ki Ho Park, Taekgeun Ohk, Jin-Won Chung, Doo Ryeon Chung, Dong Kee Kim, Sang-Rok Lee, Pill Young Kim, Shin-Woo Kim, Ji Yun Noh, Joon Young Song, Hee Jin Cheong, Woo Joo Kim
J Korean Med Sci. 2018;33(32):. doi: 10.3346/jkms.2018.33.e212.
Reference
-
1. Vaccines against influenza WHO position paper–November 2012. Wkly Epidemiol Rec. 2012; 87:461–476.2. Centers for Diseases Control and Prevention. Vaccine Recommendations of the ACIP. accessed on 2016 December 27. Available at: https://www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/flu.html.3. Wester A, Shetty AK. Peramivir injection in the treatment of acute influenza: a review of the literature. Infect Drug Resist. 2016; 9:201–214.4. Birnkrant D, Cox E. The emergency use authorization of peramivir for treatment of 2009 H1N1 influenza. N Engl J Med. 2009; 361:2204–2207.
Article5. Kohno S, Kida H, Mizuguchi M, Shimada J. S-021812 Clinical Study Group. Efficacy and safety of intravenous peramivir for treatment of seasonal influenza virus infection. Antimicrob Agents Chemother. 2010; 54:4568–4574.
Article6. Kohno S, Kida H, Mizuguchi M, Hirotsu N, Ishida T, Kadota J, et al. Intravenous peramivir for treatment of influenza A and B virus infection in high-risk patients. Antimicrob Agents Chemother. 2011; 55:2803–2812.
Article7. Ison MG, Hui DS, Clezy K, O'Neil BJ, Flynt A, Collis PJ, et al. A clinical trial of intravenous peramivir compared with oral oseltamivir for the treatment of seasonal influenza in hospitalized adults. Antivir Ther. 2013; 18:651–661.
Article8. Kohno S, Yen MY, Cheong HJ, Hirotsu N, Ishida T, Kadota J, et al. Phase III randomized, double-blind study comparing single-dose intravenous peramivir with oral oseltamivir in patients with seasonal influenza virus infection. Antimicrob Agents Chemother. 2011; 55:5267–5276.
Article9. Hikita T, Hikita H, Hikita F, Hikita N, Hikita S. Clinical effectiveness of peramivir in comparison with other neuraminidase inhibitors in pediatric influenza patients. Int J Pediatr. 2012; 2012:834181.
Article10. Shobugawa Y, Saito R, Sato I, Kawashima T, Dapat C, Dapat IC, et al. Clinical effectiveness of neuraminidase inhibitors--oseltamivir, zanamivir, laninamivir, and peramivir--for treatment of influenza A(H3N2) and A(H1N1)pdm09 infection: an observational study in the 2010-2011 influenza season in Japan. J Infect Chemother. 2012; 18:858–864.
Article11. Takemoto Y, Asai T, Ikezoe I, Yano T, Ichikawa M, Miyagawa S, et al. Clinical effects of oseltamivir, zanamivir, laninamivir and peramivir on seasonal influenza infection in outpatients in Japan during the winter of 2012-2013. Chemotherapy. 2013; 59:373–378.
Article12. Yoo JW, Choi SH, Huh JW, Lim CM, Koh Y, Hong SB. Peramivir is as effective as oral oseltamivir in the treatment of severe seasonal influenza. J Med Virol. 2015; 87:1649–1655.
Article13. Yoshino Y, Seo K, Koga I, Kitazawa T, Ota Y. Clinical efficacy of peramivir in adult patients with seasonal influenza during the winter of 2012 in Japan. Clin Respir J. 2015; 9:228–232.
Article14. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality if non-randomized studies in meta-analyses. accessed on 2016 December 27. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.15. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011; 343:d5928.
Article16. Alame MM, Massaad E, Zaraket H. Peramivir: a novel intravenous neuraminidase inhibitor for treatment of acute influenza infections. Front Microbiol. 2016; 7:450.
Article17. de Jong MD, Ison MG, Monto AS, Metev H, Clark C, O'Neil B, et al. Evaluation of intravenous peramivir for treatment of influenza in hospitalized patients. Clin Infect Dis. 2014; 59:e172–e185.
Article18. Barroso L, Treanor J, Gubareva L, Hayden FG. Efficacy and tolerability of the oral neuraminidase inhibitor peramivir in experimental human influenza: randomized, controlled trials for prophylaxis and treatment. Antivir Ther. 2005; 10:901–910.
Article19. Hata A, Akashi-Ueda R, Takamatsu K, Matsumura T. Safety and efficacy of peramivir for influenza treatment. Drug Des Devel Ther. 2014; 8:2017–2038.
Article