Yonsei Med J.
2017 Nov;58(6):1216-1221. 10.3349/ymj.2017.58.6.1216.
A Simulation Study of Propofol Effect-Site Concentration for Appropriate Sedation in Pediatric Patients Undergoing Brain MRI: Pharmacodynamic Analysis
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Anesthesia and Pain Research Institute, Yonsei University College of Medicine, Seoul, Korea. hanesth@yuhs.ac
- KMID: 2418905
- DOI: http://doi.org/10.3349/ymj.2017.58.6.1216
Abstract
- PURPOSE
We aimed to establish the propofol effect-site concentration (Ce) for appropriate sedation by pharmacodynamic analysis and to determine the propofol Ce during occurrence of sedation-related side effects in pediatric patients undergoing brain magnetic resonance imaging (MRI).
MATERIALS AND METHODS
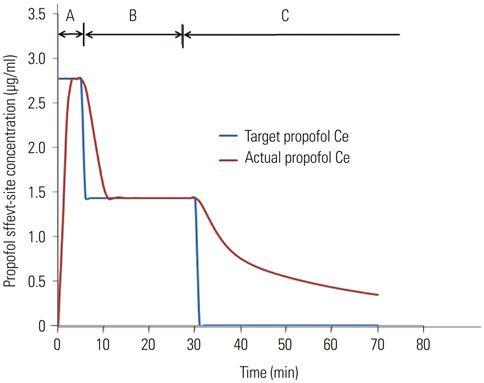
In 50 pediatric patients scheduled for brain MRI, sedation was induced with 2.0 mg/kg propofol; additional propofol doses were 0.5-1 mg/kg. Propofol Ce was simulated by inputting the propofol administration profiles of patients into a pediatric compartmental model (Choi model). The relationship between propofol Ce and probabilities of sedation and recovery were analyzed using a sigmoidal Emax model. The simulated propofol Ce for sedation-related side effects was investigated. Population model parameters were estimated using the Nonlinear Mixed-Effects Modelling software.
RESULTS
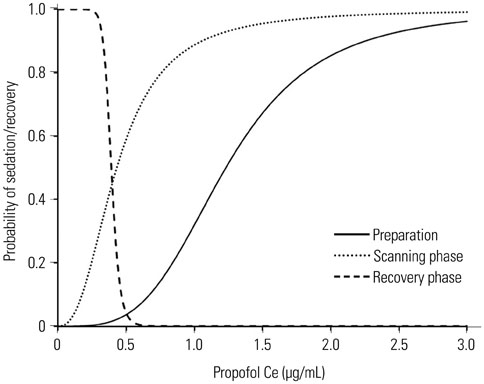
The mean values of propofol Ceâ‚…â‚€ for sedation during the preparation, scanning, and recovery phases were 1.23, 0.43, and 0.39 µg/mL. The simulated propofol Ce values during oxygen desaturation (SpOâ‚‚<90%) (3 patients; 6%), hypotension (16 patients; 32%), and bradycardia (12 patients; 24%) were 3.01±0.04, 2.05±0.63, and 2.41±0.89 µg/mL, respectively.
CONCLUSION
The required propofol Ceâ‚…â‚€ for applying monitors during the preparation phase before the start of MRI was higher than the propofol Ceâ‚…â‚€ required during the scanning phase. During low-intensity stimulation phases, such as scanning, propofol bolus dose should be strictly titrated not to exceed the propofol Ce that can lead to oxygen desaturation because of the relatively low propofol Ce (Ce₉₅, 1.43 µg/mL) required for sedation in most patients.
Keyword
MeSH Terms
-
Adolescent
Anesthesia
Anesthetics, Intravenous/administration & dosage/pharmacology
Bradycardia/complications
Brain/*diagnostic imaging
Child
Dose-Response Relationship, Drug
Female
Humans
Hypnotics and Sedatives/administration & dosage/*pharmacology
Hypotension/chemically induced
Magnetic Resonance Imaging
Male
*Models, Biological
Probability
Propofol/administration & dosage/*pharmacology
Anesthetics, Intravenous
Hypnotics and Sedatives
Propofol
Figure
Cited by 1 articles
-
Sedation Strategies for Procedures Outside the Operating Room
Youn Yi Jo, Hyun Jeong Kwak
Yonsei Med J. 2019;60(6):491-499. doi: 10.3349/ymj.2019.60.6.491.
Reference
-
1. Mark A, Brown RCS. Magnetic resonance imaging principles and applications. In : Joseph KTL, Stuart SS, Robert JS, Jay PH, editors. Computed body tomography with MRI correlation. 4th ed. Philadelphia: Lippincott Williams & Wilkins;2006. p. 29–93.2. Hasan RA, Shayevitz JR, Patel V. Deep sedation with propofol for children undergoing ambulatory magnetic resonance imaging of the brain: experience from a pediatric intensive care unit. Pediatr Crit Care Med. 2003; 4:454–458.
Article3. Serafini G, Zadra N. Anaesthesia for MRI in the paediatric patient. Curr Opin Anaesthesiol. 2008; 21:499–503.
Article4. Sury MR, Smith JH. Deep sedation and minimal anesthesia. Paediatr Anaesth. 2008; 18:18–24.
Article5. Vangerven M, Van Hemelrijck J, Wouters P, Vandermeersch E, Van Aken H. Light anaesthesia with propofol for paediatric MRI. Anaesthesia. 1992; 47:706–707.
Article6. Cravero JP, Beach ML, Blike GT, Gallagher SM, Hertzog JH. Pediatric Sedation Research Consortium. The incidence and nature of adverse events during pediatric sedation/anesthesia with propofol for procedures outside the operating room: a report from the Pediatric Sedation Research Consortium. Anesth Analg. 2009; 108:795–804.
Article7. Mallory MD, Baxter AL, Kost SI. Pediatric Sedation Research Consortium. Propofol vs pentobarbital for sedation of children undergoing magnetic resonance imaging: results from the Pediatric Sedation Research Consortium. Paediatr Anaesth. 2009; 19:601–611.
Article8. Pershad J, Wan J, Anghelescu DL. Comparison of propofol with pentobarbital/midazolam/fentanyl sedation for magnetic resonance imaging of the brain in children. Pediatrics. 2007; 120:e629–e636.
Article9. Machata AM, Willschke H, Kabon B, Kettner SC, Marhofer P. Propofol-based sedation regimen for infants and children undergoing ambulatory magnetic resonance imaging. Br J Anaesth. 2008; 101:239–243.
Article10. Kitt E, Friderici J, Kleppel R, Canarie M. Procedural sedation for MRI in children with ADHD. Paediatr Anaesth. 2015; 25:1026–1032.
Article11. American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non-Anesthesiologists. Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology. 2002; 96:1004–1017.12. Aldrete JA, Kroulik D. A postanesthetic recovery score. Anesth Analg. 1970; 49:924–934.
Article13. Choi BM, Lee HG, Byon HJ, Lee SH, Lee EK, Kim HS, et al. Population pharmacokinetic and pharmacodynamic model of propofol externally validated in children. J Pharmacokinet Pharmacodyn. 2015; 42:163–177.
Article14. Lu W, Ramsay JG, Bailey JM. Reliability of pharmacodynamic analysis by logistic regression: mixed-effects modeling. Anesthesiology. 2003; 99:1255–1262.15. Srinivasan M, Turmelle M, Depalma LM, Mao J, Carlson DW. Procedural sedation for diagnostic imaging in children by pediatric hospitalists using propofol: analysis of the nature, frequency, and predictors of adverse events and interventions. J Pediatr. 2012; 160:801–806.e1.
Article16. Schulte-Uentrop L, Goepfert MS. Anaesthesia or sedation for MRI in children. Curr Opin Anaesthesiol. 2010; 23:513–517.
Article17. Koroglu A, Teksan H, Sagir O, Yucel A, Toprak HI, Ersoy OM. A comparison of the sedative, hemodynamic, and respiratory effects of dexmedetomidine and propofol in children undergoing magnetic resonance imaging. Anesth Analg. 2006; 103:63–67.
Article18. Gutmann A, Pessenbacher K, Gschanes A, Eggenreich U, Wargenau M, Toller W. Propofol anesthesia in spontaneously breathing children undergoing magnetic resonance imaging: comparison of two propofol emulsions. Paediatr Anaesth. 2006; 16:266–274.
Article19. Krauss B, Green SM. Sedation and analgesia for procedures in children. N Engl J Med. 2000; 342:938–945.
Article20. Koo BN, Shin S, Kim SY, Kang YR, Jeong KH, Han DW. Pharmacodynamic estimate of propofol-induced sedation and airway obstruction effects in obstructive sleep apnea-hypopnea syndrome. Yonsei Med J. 2015; 56:1408–1414.
Article21. Milius EM, Papademetrious TR, Heitlinger LA. Retrospective review of propofol dosing for procedural sedation in pediatric patients. J Pediatr Pharmacol Ther. 2012; 17:246–251.
Article22. Kim EJ, Jo YY, Kil HK. Optimal sedative dose of propofol to start MRI in children with cerebral palsy. Korean J Anesthesiol. 2011; 61:216–219.
Article23. Arthurs OJ, Sury M. Anaesthesia or sedation for paediatric MRI: advantages and disadvantages. Curr Opin Anaesthesiol. 2013; 26:489–494.24. Osborn IP. Magnetic resonance imaging anesthesia: new challenges and techniques. Curr Opin Anaesthesiol. 2002; 15:443–448.
Article25. Gozal D, Mason KP. Pediatric sedation: a global challenge. Int J Pediatr. 2010; 2010:701257.
Article26. Rigouzzo A, Girault L, Louvet N, Servin F, De-Smet T, Piat V, et al. The relationship between bispectral index and propofol during target-controlled infusion anesthesia: a comparative study between children and young adults. Anesth Analg. 2008; 106:1109–1116.
Article27. Constant I, Rigouzzo A. Which model for propofol TCI in children. Paediatr Anaesth. 2010; 20:233–239.
Article28. McClune S, McKay AC, Wright PM, Patterson CC, Clarke RS. Synergistic interaction between midazolam and propofol. Br J Anaesth. 1992; 69:240–245.
Article29. Olmos M, Ballester JA, Vidarte MA, Elizalde JL, Escobar A. The combined effect of age and premedication on the propofol requirements for induction by target-controlled infusion. Anesth Analg. 2000; 90:1157–1161.
Article30. Sanborn PA, Michna E, Zurakowski D, Burrows PE, Fontaine PJ, Connor L, et al. Adverse cardiovascular and respiratory events during sedation of pediatric patients for imaging examinations. Radiology. 2005; 237:288–294.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Titration of Effect Site Concentration of Propofol for Conscious Sedation in Elderly Patients
- Development of an Adequate Propofol Sedation Model in Elderly Patients for Elective Surgery under Regional Anesthesia
- Obesity and anesthetic pharmacology: simulation of target-controlled infusion models of propofol and remifentanil
- Repeated Sedation with Intravenous Propofol in a Brain Tumor Patient during ConsecutiveRadiation Therapy : A case report
- Correlations between the Modified Observer's Assessment of Alertness/Sedation Scale, Bispectral Index and Propofol Effect Site Concentrations in Sedated Elderly Patients under Regional Anesthesia