Yonsei Med J.
2017 Nov;58(6):1101-1110. 10.3349/ymj.2017.58.6.1101.
Overexpression of miR-191 Predicts Poor Prognosis and Promotes Proliferation and Invasion in Esophageal Squamous Cell Carcinoma
- Affiliations
-
- 1Department of Cardiac Surgery, The First Affiliated Hospital of Anhui Medical University, Hefei, China.
- 2Department of Cardiothoracic Surgery, Affiliated Hospital of Guangdong Medical University, Zhanjiang, China.
- 3Department of Internal Medicine, Guangdong Medical University Affiliated Longhua Central Hospital, Shenzhen, China. 1347240737@qq.com
- KMID: 2418891
- DOI: http://doi.org/10.3349/ymj.2017.58.6.1101
Abstract
- PURPOSE
Accumulating evidence has shown that dysregulation of microRNA-191 (miR-191) is closely associated with tumorigenesis and progression in a wide range of cancers. This study aimed to explore the potential role of miR-191 in esophageal squamous cell carcinoma (ESCC).
MATERIALS AND METHODS
miR-191 expression was assessed in 93 ESCC tissue specimens by real-time polymerase chain reaction, and survival analysis was performed via Kaplan-Meier and Cox regression analyses. 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide, plate colony-forming, BrdU, and Transwell assays were conducted to observe the effect of miR-191 on ESCC proliferation and invasion. Luciferase reporter and western blot assays were taken to identify target genes of miR-191.
RESULTS
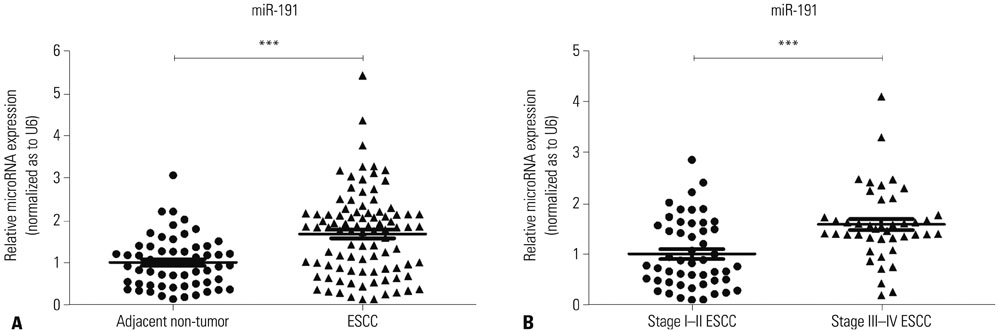
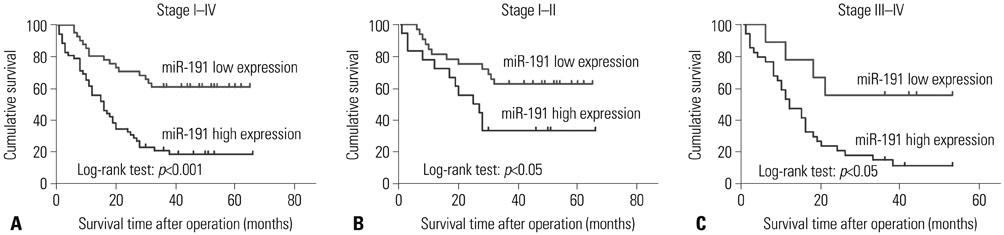
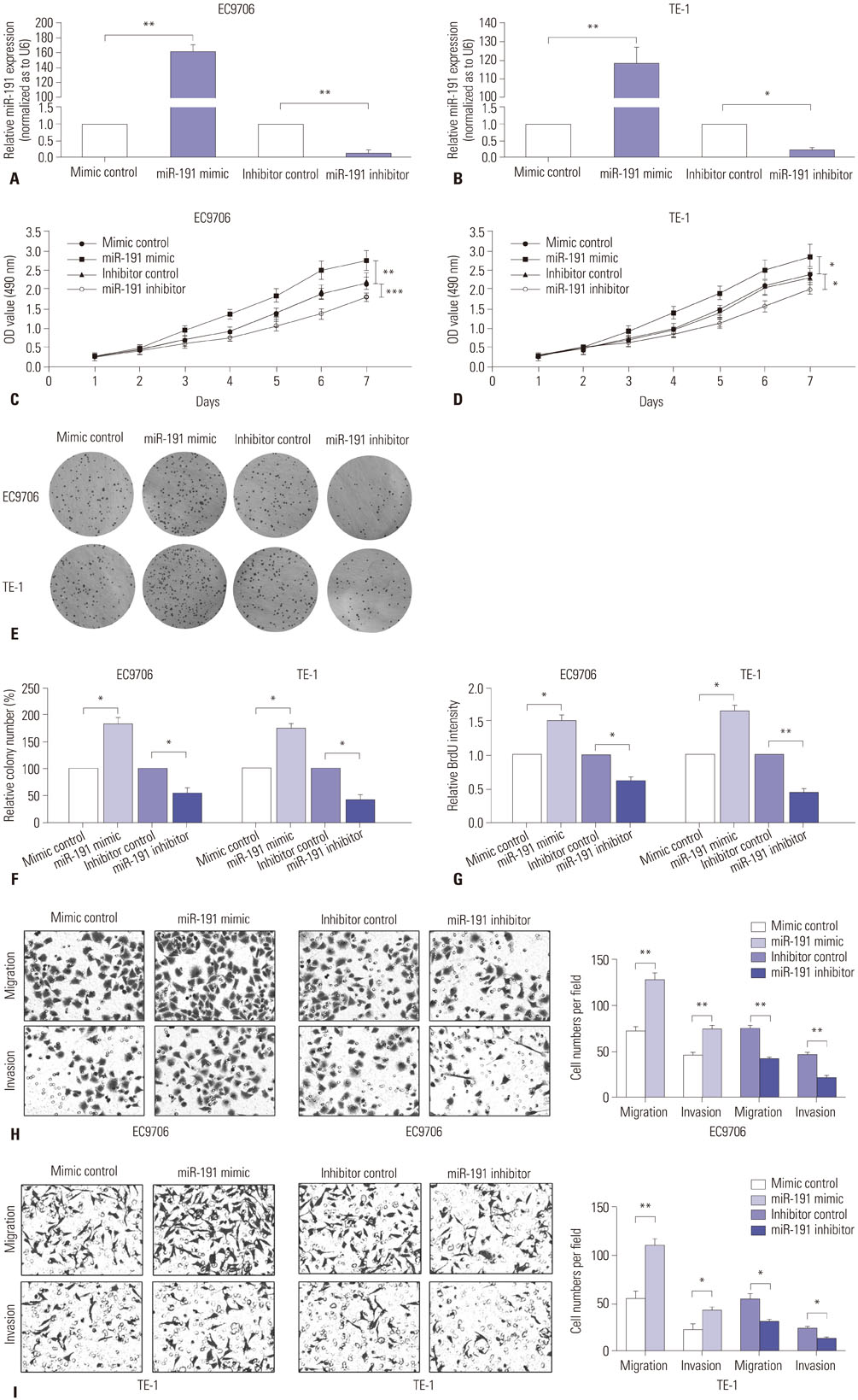
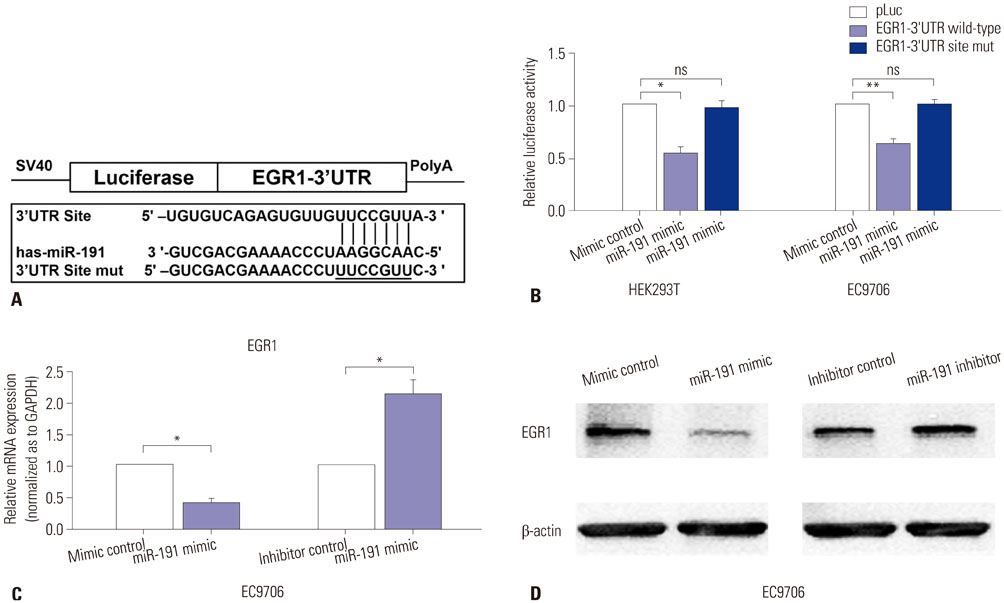
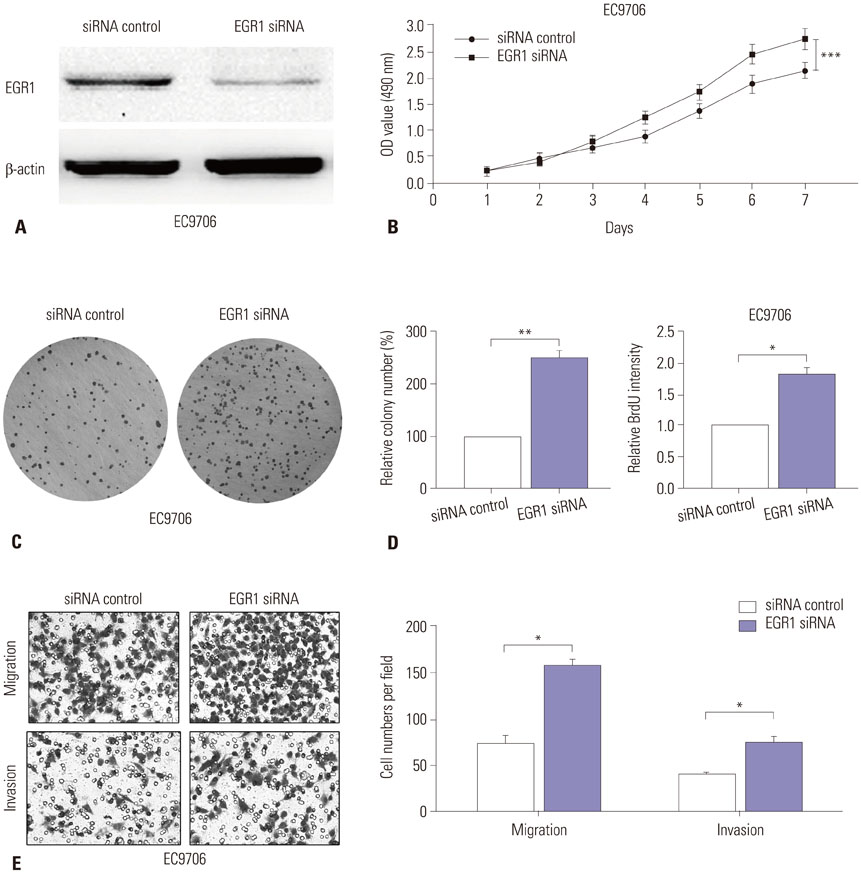
miR-191 was overexpressed in 93 cases of ESCC, compared with adjacent normal tissues, and miR-191 expression was significantly related to differentiation, depth of invasion, TNM stage, lymph node metastasis, and distant metastasis of tumor. Kaplan-Meier and Cox regression analyses demonstrated that overexpression of miR-191 was an independent and significant predictor of ESCC prognosis. Both gain-of-function and loss-of-function experiments showed that miR-191 promoted ESCC cell proliferation and invasion activities in vitro. Early growth response 1 (EGR1), a tumor suppressor, was predicted as a direct target of miR-191. Luciferase reporter and western blot assays proved that miR-191 reduced EGR1 expression by directly binding its 3' untranslated region. Moreover, EGR1 knockdown by siRNA enhanced ESCC cell growth and invasion.
CONCLUSION
Our findings provide specific biological roles of miR-191 in ESCC survival and progression. Targeting the novel miR-191/EGR1 axis represents a potential new therapeutic way to block ESCC development.
Keyword
MeSH Terms
-
3' Untranslated Regions
Carcinoma, Squamous Cell/genetics/metabolism/*pathology
Cell Line, Tumor
*Cell Movement
Cell Proliferation
Esophageal Neoplasms/genetics/metabolism/*pathology
*Gene Expression Regulation, Neoplastic
Humans
Lymphatic Metastasis
MicroRNAs/*genetics/metabolism
Middle Aged
Neoplasm Invasiveness/*genetics
Prognosis
RNA, Small Interfering
Real-Time Polymerase Chain Reaction
3' Untranslated Regions
MicroRNAs
RNA, Small Interfering
Figure
Reference
-
1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017; 67:7–30.
Article2. Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003; 349:2241–2252.
Article3. Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013; 381:400–412.
Article4. Yates LA, Norbury CJ, Gilbert RJ. The long and short of microRNA. Cell. 2013; 153:516–519.
Article5. Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009; 136:215–233.
Article6. Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 2006; 6:259–269.
Article7. Nagpal N, Kulshreshtha R. miR-191: an emerging player in disease biology. Front Genet. 2014; 5:99.
Article8. Dangwal S, Stratmann B, Bang C, Lorenzen JM, Kumarswamy R, Fiedler J, et al. Impairment of wound healing in patients with type 2 diabetes mellitus influences circulating microRNA patterns via inflammatory cytokines. Arterioscler Thromb Vasc Biol. 2015; 35:1480–1488.
Article9. Tan L, Yu JT, Tan MS, Liu QY, Wang HF, Zhang W, et al. Genomewide serum microRNA expression profiling identifies serum biomarkers for Alzheimer's disease. J Alzheimers Dis. 2014; 40:1017–1027.
Article10. Wei C, Henderson H, Spradley C, Li L, Kim IK, Kumar S. . Circulating miRNAs as potential marker for pulmonary hypertension. PLoS One. 2013; 05. 23. DOI: 10.1371/journal.pone.0064396. [Epub].
Article11. Paraskevi A, Theodoropoulos G, Papaconstantinou I, Mantzaris G, Nikiteas N, Gazouli M. Circulating MicroRNA in inflammatory bowel disease. J Crohns Colitis. 2012; 6:900–904.
Article12. Nagpal N, Ahmad HM, Molparia B, Kulshreshtha R. MicroRNA-191, an estrogen-responsive microRNA, functions as an oncogenic regulator in human breast cancer. Carcinogenesis. 2013; 34:1889–1899.
Article13. Zhang XF, Li KK, Gao L, Li SZ, Chen K, Zhang JB, et al. miR-191 promotes tumorigenesis of human colorectal cancer through targeting C/EBPβ. Oncotarget. 2015; 6:4144–4158.
Article14. Xu W, Ji J, Xu Y, Liu Y, Shi L, Liu Y, et al. MicroRNA-191, by promoting the EMT and increasing CSC-like properties, is involved in neoplastic and metastatic properties of transformed human bronchial epithelial cells. Mol Carcinog. 2015; 54:Suppl 1. E148–E161.
Article15. McEvoy J, Ulyanov A, Brennan R, Wu G, Pounds S, Zhang J, et al. Analysis of MDM2 and MDM4 single nucleotide polymorphisms, mRNA splicing and protein expression in retinoblastoma. PLoS One. 2012; 7:e42739.
Article16. Colamaio M, Borbone E, Russo L, Bianco M, Federico A, Califano D, et al. miR-191 down-regulation plays a role in thyroid follicular tumors through CDK6 targeting. J Clin Endocrinol Metab. 2011; 96:E1915–E1924.
Article17. Xiao D, Barry S, Kmetz D, Egger M, Pan J, Rai SN, et al. Melanoma cell-derived exosomes promote epithelial-mesenchymal transition in primary melanocytes through paracrine/autocrine signaling in the tumor microenvironment. Cancer Lett. 2016; 376:318–327.
Article18. Peng WZ, Ma R, Wang F, Yu J, Liu ZB. Role of miR-191/425 cluster in tumorigenesis and diagnosis of gastric cancer. Int J Mol Sci. 2014; 15:4031–4048.
Article19. Yuan L, Zhang Y, Xia J, Liu B, Zhang Q, Liu J, et al. Resveratrol induces cell cycle arrest via a p53-independent pathway in A549 cells. Mol Med Rep. 2015; 11:2459–2464.
Article20. Harada K, Baba Y, Ishimoto T, Shigaki H, Kosumi K, Yoshida N, et al. The role of microRNA in esophageal squamous cell carcinoma. J Gastroenterol. 2016; 51:520–530.
Article21. Komatsu S, Ichikawa D, Kawaguchi T, Miyamae M, Okajima W, Ohashi T, et al. Circulating miR-21 as an independent predictive biomarker for chemoresistance in esophageal squamous cell carcinoma. Am J Cancer Res. 2016; 6:1511–1523.22. Liu R, Gu J, Jiang P, Zheng Y, Liu X, Jiang X, et al. DNMT1-microRNA126 epigenetic circuit contributes to esophageal squamous cell carcinoma growth via ADAM9-EGFR-AKT signaling. Clin Cancer Res. 2015; 21:854–863.
Article23. Gong H, Song L, Lin C, Liu A, Lin X, Wu J, et al. Downregulation of miR-138 sustains NF-κB activation and promotes lipid raft formation in esophageal squamous cell carcinoma. Clin Cancer Res. 2013; 19:1083–1093.
Article24. Yang M, Liu R, Li X, Liao J, Pu Y, Pan E, et al. miRNA-183 suppresses apoptosis and promotes proliferation in esophageal cancer by targeting PDCD4. Mol Cells. 2014; 37:873–880.
Article25. Zhang HF, Alshareef A, Wu C, Jiao JW, Sorensen PH, Lai R, et al. miR-200b induces cell cycle arrest and represses cell growth in esophageal squamous cell carcinoma. Carcinogenesis. 2016; 37:858–869.
Article26. Osako Y, Seki N, Kita Y, Yonemori K, Koshizuka K, Kurozumi A, et al. Regulation of MMP13 by antitumor microRNA-375 markedly inhibits cancer cell migration and invasion in esophageal squamous cell carcinoma. Int J Oncol. 2016; 49:2255–2264.
Article27. Lin C, Liu A, Zhu J, Zhang X, Wu G, Ren P, et al. miR-508 sustains phosphoinositide signalling and promotes aggressive phenotype of oesophageal squamous cell carcinoma. Nat Commun. 2014; 5:4620.
Article28. Elyakim E, Sitbon E, Faerman A, Tabak S, Montia E, Belanis L, et al. hsa-miR-191 is a candidate oncogene target for hepatocellular carcinoma therapy. Cancer Res. 2010; 70:8077–8087.
Article29. Di Leva G, Piovan C, Gasparini P, Ngankeu A, Taccioli C, Briskin D, et al. Estrogen mediated-activation of miR-191/425 cluster modulates tumorigenicity of breast cancer cells depending on estrogen receptor status. PLoS Genet. 2013; 9:e1003311.
Article30. Qin S, Zhu Y, Ai F, Li Y, Bai B, Yao W, et al. MicroRNA-191 correlates with poor prognosis of colorectal carcinoma and plays multiple roles by targeting tissue inhibitor of metalloprotease 3. Neoplasma. 2014; 61:27–34.
Article31. Ferraro B, Bepler G, Sharma S, Cantor A, Haura EB. EGR1 predicts PTEN and survival in patients with non-small-cell lung cancer. J Clin Oncol. 2005; 23:1921–1926.
Article32. Sarver AL, Li L, Subramanian S. MicroRNA miR-183 functions as an oncogene by targeting the transcription factor EGR1 and promoting tumor cell migration. Cancer Res. 2010; 70:9570–9580.
Article33. Liu C, Adamson E, Mercola D. Transcription factor EGR-1 suppresses the growth and transformation of human HT-1080 fibrosarcoma cells by induction of transforming growth factor beta 1. Proc Natl Acad Sci U S A. 1996; 93:11831–11836.
Article34. Wu MY, Wu XY, Li QS, Zheng RM. Expression of Egr-1 gene and its correlation with the oncogene proteins in non-irradiated and irradiated esophageal squamous cell carcinoma. Dis Esophagus. 2006; 19:267–272.
Article35. Dong Q, Zhang J, Hendricks DT, Zhao X. GROβ and its downstream effector EGR1 regulate cisplatin-induced apoptosis in WHCO1 cells. Oncol Rep. 2011; 25:1031–1037.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- LINC00662 Promotes Oral Squamous Cell Carcinoma Cell Growth and Metastasis through miR-144-3p/EZH2 Axis
- LncRNA DLG1-AS1 Promotes Cancer Cell Proliferation in Triple Negative Breast Cancer by Downregulating miR-203
- MiR-152 suppresses the proliferation and invasion of NSCLC cells by inhibiting FGF2
- Effects of p27 Overexpression on Head and Neck Squamous Cell Carcinoma Cell Lines
- MicroRNA-373 Inhibits Cell Proliferation and Invasion via Targeting BRF2 in Human Non-small Cell Lung Cancer A549 Cell Line