Korean Circ J.
2018 Sep;48(9):839-853. 10.4070/kcj.2017.0394.
Hemodynamic and Histopathologic Benefits of Early Treatment with Macitentan in a Rat Model of Pulmonary Arterial Hypertension
- Affiliations
-
- 1Division of Cardiology, Department of Internal Medicine, Sejong General Hospital, Bucheon, Korea.
- 2Division of Cardiology, Department of Internal Medicine, Cardiovascular Center, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea. cardiman73@gmail.com
- 3Pittsburgh Heart, Lung, Blood, and Vascular Medicine Institute, Division of Cardiology, Department of Medicine, University of Pittsburgh Medical Center (UPMC) and University of Pittsburgh School of Medicine, Pittsburgh, PA, USA.
- KMID: 2418810
- DOI: http://doi.org/10.4070/kcj.2017.0394
Abstract
- BACKGROUND AND OBJECTIVES
Macitentan (MAC) reduces morbidity and mortality among advanced-stage pulmonary arterial hypertension (PAH) patients. However, data regarding the histopathologic and hemodynamic benefits of MAC treatment at an early stage of PAH is lacking.
METHODS
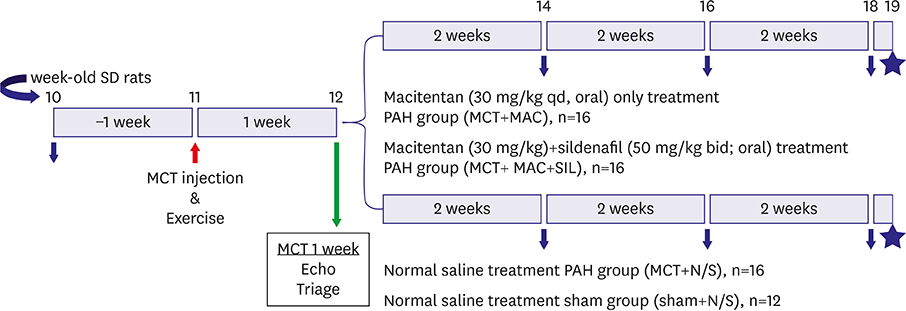
One week after monocrotaline (MCT) injection, rats were randomly assigned to MAC (n=16), MAC combined with sildenafil (SIL) (MAC+SIL, n=16), or normal saline (MCT, n=16). Twelve sham rats (Sham) were included for comparison. Right ventricular (RV) systolic function was assessed via echocardiography as the RV fractional area change (RV-FAC). An invasive pressure-volume analysis using a Millar conductance catheter was performed 7 weeks after MCT injection. Rats were subsequently euthanized for histopathologic analysis.
RESULTS
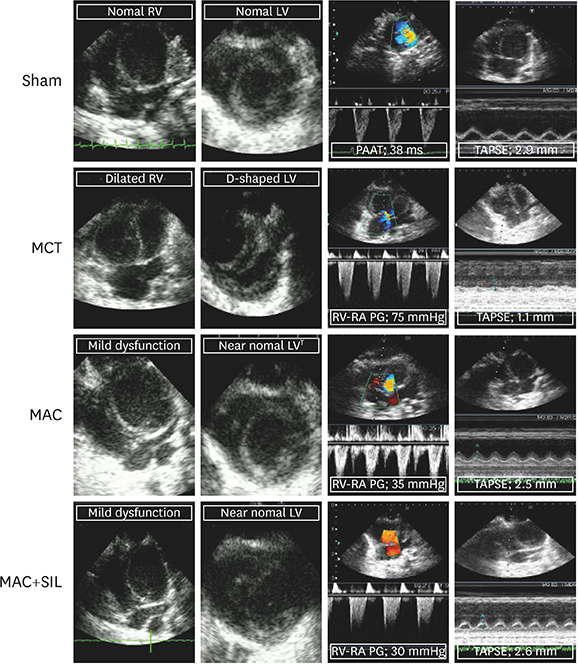
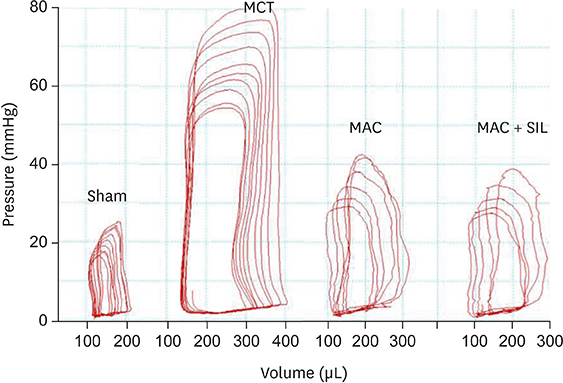
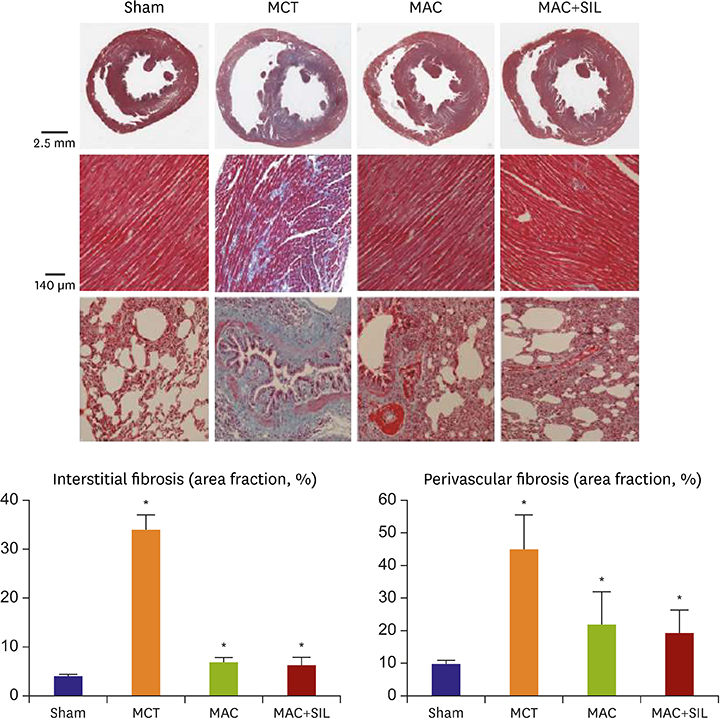
RV-right atrial pressure gradient on echocardiography was significantly increased 3 weeks after MCT injection, but was maintained in the Sham. RV-FAC was less deteriorated in the MAC, compared to that in the MCT (44±3% vs. 25±7%, p < 0.05), and the co-administration of SIL showed no additional benefit (45±8%, p > 0.05 vs. the MAC). On invasive hemodynamic analyses, RV end-systolic (196±78 µL) and end-diastolic volumes (310±86 µL), pulmonary artery systolic pressure (89±7.2 mmHg), and end-systolic pressure-volume relationship (−254±25.1) were significantly worse in the MCT vs. in the MAC (101±45 µL, 235±55 µL, 40±10.5 mmHg, and −145±42.1, respectively) and MAC+SIL (109±47 µL, 242±46 µL, 38±9.2 mmHg, and −151±39.2, respectively) (all p < 0.05). However, the MAC and MAC+SIL did not differ (all p > 0.05). On histopathology, both RV and lung fibrosis were significantly reduced in the MAC and MAC+SIL vs. in the MCT (all p < 0.05); the 2 treatment groups did not differ.
CONCLUSIONS
MAC treatment at an earlier stage significantly attenuated experimental PAH progression hemodynamically and histopathologically.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Benefits from the Early Initiation of Macitentan for Pulmonary Arterial Hypertension
Hae Ok Jung
Korean Circ J. 2018;48(9):854-856. doi: 10.4070/kcj.2018.0156.Moving Beyond the Endothelium is Still Challenging-Complex Interplay between Endothelin and Reactive Oxygen Species in Pulmonary Arterial Hypertension
Hyemoon Chung, Il Suk Sohn
Korean Circ J. 2019;49(9):877-878. doi: 10.4070/kcj.2019.0167.
Reference
-
1. Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016; 37:67–119.2. Humbert M, Sitbon O, Chaouat A, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation. 2010; 122:156–163.
Article3. Schermuly RT, Ghofrani HA, Wilkins MR, Grimminger F. Mechanisms of disease: pulmonary arterial hypertension. Nat Rev Cardiol. 2011; 8:443–455.
Article4. Nogueira-Ferreira R, Vitorino R, Ferreira R, Henriques-Coelho T. Exploring the monocrotaline animal model for the study of pulmonary arterial hypertension: a network approach. Pulm Pharmacol Ther. 2015; 35:8–16.
Article5. Galiè N, Rubin L, Hoeper M, et al. Treatment of patients with mildly symptomatic pulmonary arterial hypertension with bosentan (EARLY study): a double-blind, randomised controlled trial. Lancet. 2008; 371:2093–2100.
Article6. Pulido T, Adzerikho I, Channick RN, et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med. 2013; 369:809–818.
Article7. Galiè N, Ghofrani HA, Torbicki A, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005; 353:2148–2157.
Article8. Kim KH, Kim YJ, Ohn JH, et al. Long-term effects of sildenafil in a rat model of chronic mitral regurgitation: benefits of ventricular remodeling and exercise capacity. Circulation. 2012; 125:1390–1401.9. Takimoto E, Champion HC, Li M, et al. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med. 2005; 11:214–222.
Article10. Stenmark KR, Meyrick B, Galie N, Mooi WJ, McMurtry IF. Animal models of pulmonary arterial hypertension: the hope for etiological discovery and pharmacological cure. Am J Physiol Lung Cell Mol Physiol. 2009; 297:L1013–L1032.
Article11. Baber SR, Deng W, Master RG, et al. Intratracheal mesenchymal stem cell administration attenuates monocrotaline-induced pulmonary hypertension and endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2007; 292:H1120–H1128.
Article12. Hessel MH, Steendijk P, den Adel B, Schutte CI, van der Laarse A. Characterization of right ventricular function after monocrotaline-induced pulmonary hypertension in the intact rat. Am J Physiol Heart Circ Physiol. 2006; 291:H2424–H2430.
Article13. Ding M, Lei J, Qu Y, et al. Calorie restriction attenuates monocrotaline-induced pulmonary arterial hypertension in rats. J Cardiovasc Pharmacol. 2015; 65:562–570.14. Iglarz M, Bossu A, Wanner D, et al. Comparison of pharmacological activity of macitentan and bosentan in preclinical models of systemic and pulmonary hypertension. Life Sci. 2014; 118:333–339.
Article15. Iglarz M, Binkert C, Morrison K, et al. Pharmacology of macitentan, an orally active tissue-targeting dual endothelin receptor antagonist. J Pharmacol Exp Ther. 2008; 327:736–745.
Article16. Clozel M, Hess P, Rey M, Iglarz M, Binkert C, Qiu C. Bosentan, sildenafil, and their combination in the monocrotaline model of pulmonary hypertension in rats. Exp Biol Med (Maywood). 2006; 231:967–973.17. Kim KH, Kim YJ, Lee SP, et al. Survival, exercise capacity, and left ventricular remodeling in a rat model of chronic mitral regurgitation: serial echocardiography and pressure-volume analysis. Korean Circ J. 2011; 41:603–611.
Article18. Tyndall AJ, Bannert B, Vonk M, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis. 2010; 69:1809–1815.19. Condliffe R, Kiely DG, Peacock AJ, et al. Connective tissue disease-associated pulmonary arterial hypertension in the modern treatment era. Am J Respir Crit Care Med. 2009; 179:151–157.
Article20. Rubin LJ, Badesch DB, Barst RJ, et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med. 2002; 346:896–903.
Article21. Galié N, Badesch D, Oudiz R, et al. Ambrisentan therapy for pulmonary arterial hypertension. J Am Coll Cardiol. 2005; 46:529–535.
Article22. Iglarz M, Landskroner K, Bauer Y, et al. Comparison of macitentan and bosentan on right ventricular remodeling in a rat model of non-vasoreactive pulmonary hypertension. J Cardiovasc Pharmacol. 2015; 66:457–467.
Article23. Bruderer S, Hopfgartner G, Seiberling M, et al. Absorption, distribution, metabolism, and excretion of macitentan, a dual endothelin receptor antagonist, in humans. Xenobiotica. 2012; 42:901–910.
Article24. Galiè N, Barberà JA, Frost AE, et al. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med. 2015; 373:834–844.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Benefits from the Early Initiation of Macitentan for Pulmonary Arterial Hypertension
- Updated clinical classification of pulmonary hypertension
- Pulmonary Arterial Hypertension
- Diagnosis and treatment of idiopathic pulmonary arterial hypertension
- Pulmonary Arterial Hypertension with Congenital Heart Diseases