Anat Cell Biol.
2018 Mar;51(1):52-61. 10.5115/acb.2018.51.1.52.
Ameliorative effect of vitamin B12 on seminiferous epithelium of cimetidine-treated rats: a histopathological, immunohistochemical and ultrastructural study
- Affiliations
-
- 1Department of Anatomy and Embryology, Faculty of Medicine, Mansoura University, Mansoura, Egypt. meladl@sharjah.ac.ae
- 2Department of Anatomy and Embryology, Faculty of Medicine, Taibah University, Madinah, Saudi Arabia.
- 3Department of Basic Medical Sciences, College of Medicine, University of Sharjah, Sharjah, UAE.
- KMID: 2418395
- DOI: http://doi.org/10.5115/acb.2018.51.1.52
Abstract
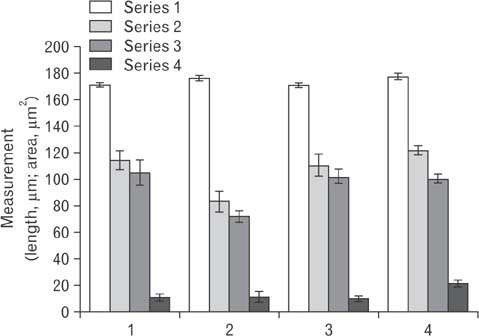
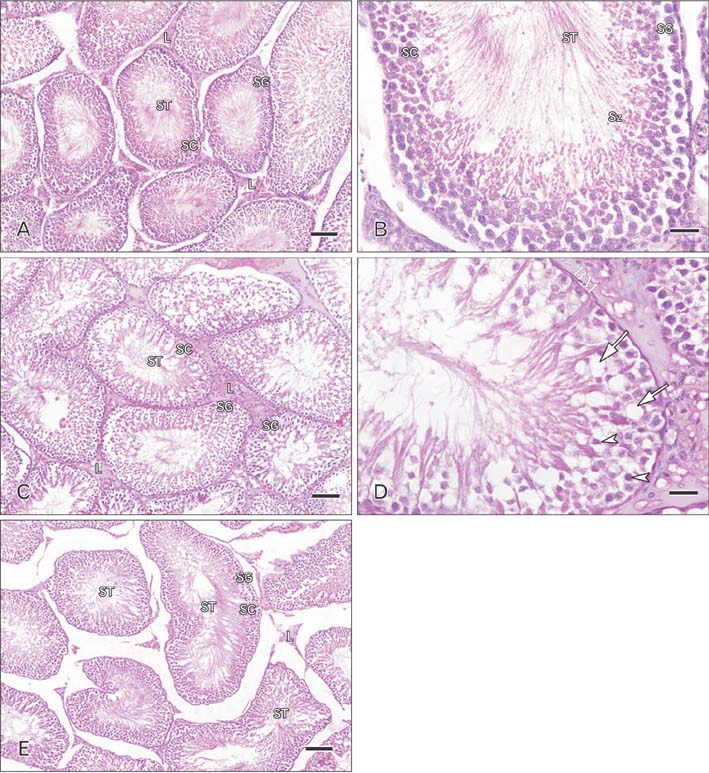
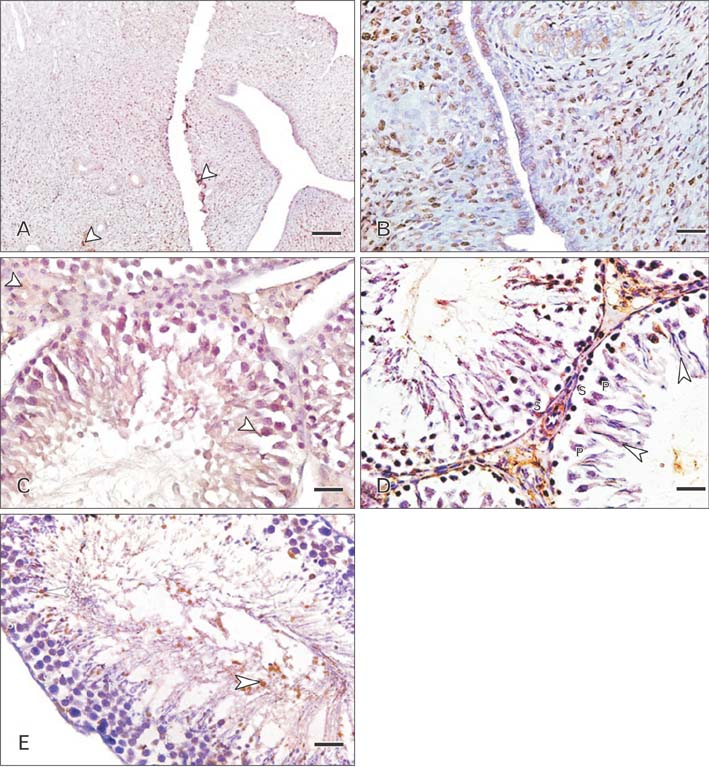
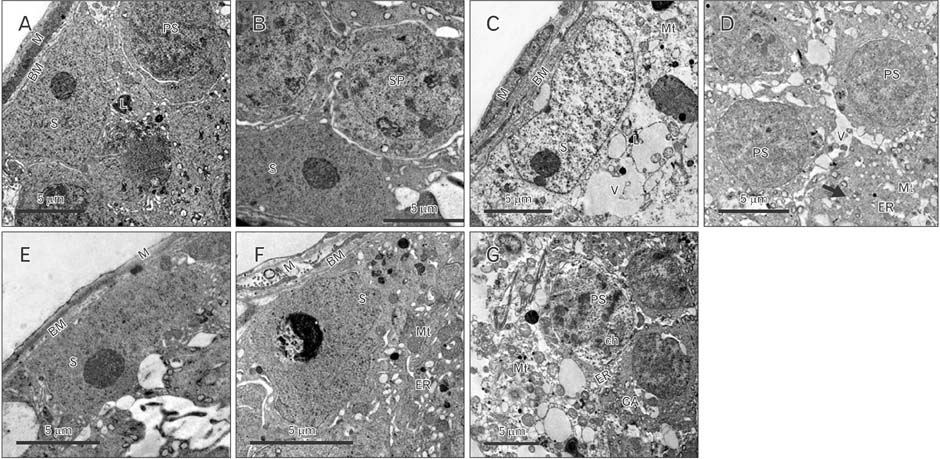
- Cimetidine is an H2 receptor antagonist that has an antiandrogenic effect. It intervenes with the conversion of testosterone into estrogen in the Sertoli cells with accompanying testicular structural changes. In the present study, the microscopic and the ultrastructural changes induced by cimetidine and the effect of vitamin B12 as a protective agent on rat testes were studied. Immunoexpression of estrogen receptor β (ERβ) in testes was evaluated. Twenty-four adult male rats were divided into four groups: control, cimetidine-treated, vitamin B12 treated, and combined cimetidine and vitamin B12 treated. The experimental rats were administered with cimetidine and/or vitamin B12 for 52 days. Group II rats showed marked atrophy of the seminiferous tubules with a significant increase in tubular diameter and decrease in the tubular luminal and epithelial areas. Ultrastructure of this group showed irregular Sertoli cells with basal cytoplasmic vacuolation and significantly thickened basement membrane. ERβ immunoexpression was similar to controls. Group III rats showed near normal seminiferous tubular structures with minimal cellular alterations and the immunoreactivity of the testicular sections was very close to normal. However, group IV rats showed markedly immunopositive detached cells, spermatids, and primary spermatocytes. Cimetidine interferes with the control of spermatogenesis as evidenced by microscopic and ultrastructural studies and affection of ERβ receptors and vitamin B12 has a protective action against this harmful effect.
Keyword
MeSH Terms
Figure
Reference
-
1. Kubecova M, Kolostova K, Pinterova D, Kacprzak G, Bobek V. Cimetidine: an anticancer drug? Eur J Pharm Sci. 2011; 42:439–444.
Article2. Kalra BS, Aggarwal S, Khurana N, Gupta U. Effect of cimetidine on hepatotoxicity induced by isoniazid-rifampicin combination in rabbits. Indian J Gastroenterol. 2007; 26:18–21.3. Beltrame FL, Caneguim BH, Miraglia SM, Cerri PS, Sasso-Cerri E. Vitamin B12 supplement exerts a beneficial effect on the seminiferous epithelium of cimetidine-treated rats. Cells Tissues Organs. 2011; 193:184–194.4. Sasso-Cerri E. Enhanced ERbeta immunoexpression and apoptosis in the germ cells of cimetidine-treated rats. Reprod Biol Endocrinol. 2009; 7:127.
Article5. Genissel C, Levallet J, Carreau S. Regulation of cytochrome P450 aromatase gene expression in adult rat Leydig cells: comparison with estradiol production. J Endocrinol. 2001; 168:95–105.
Article6. McCullough LD, Blizzard K, Simpson ER, Oz OK, Hurn PD. Aromatase cytochrome P450 and extragonadal estrogen play a role in ischemic neuroprotection. J Neurosci. 2003; 23:8701–8705.
Article7. Rago V, Maggiolini M, Vivacqua A, Palma A, Carpino A. Differential expression of estrogen receptors (ERalpha/ERbeta) in testis of mature and immature pigs. Anat Rec A Discov Mol Cell Evol Biol. 2004; 281:1234–1239.8. Hamid Q, Hamid S, Minhas LA, Gul A. Influence of cimetidine and bromocriptine on prolactin levels in rat fertility. Int J Physiol Pathophysiol Pharmacol. 2009; 1:33–40.9. Oh R, Brown DL. Vitamin B12 deficiency. Am Fam Physician. 2003; 67:979–986.10. Ruscin JM, Page RL 2nd, Valuck RJ. Vitamin B(12) deficiency associated with histamine(2)-receptor antagonists and a protonpump inhibitor. Ann Pharmacother. 2002; 36:812–816.
Article11. Hata K, Okano M, Lei H, Li E. Dnmt3L cooperates with the Dnmt3 family of de novo DNA methyltransferases to establish maternal imprints in mice. Development. 2002; 129:1983–1993.
Article12. Yamada K, Kawata T, Wada M, Mori K, Tamai H, Tanaka N, Tadokoro T, Tobimatsu T, Toraya T, Maekawa A. Testicular injury to rats fed on soybean protein-based vitamin B12-deficient diet can be reduced by methionine supplementation. J Nutr Sci Vitaminol (Tokyo). 2007; 53:95–101.
Article13. Kawata T, Funada U, Wada M, Matsushita M, Sanai T, Yamada H, Kuwamori M, Arai K, Yamamoto Y, Tanaka N, Tadokoro T, Maekawa A. Breeding severely vitamin B12-deficient mice as model animals. Int J Vitam Nutr Res. 2004; 74:57–63.
Article14. Oshio S, Ozaki S, Ohkawa I, Tajima T, Kaneko S, Mohri H. Mecobalamin promotes mouse sperm maturation. Andrologia. 1989; 21:167–173.15. Rago V, Siciliano L, Aquila S, Carpino A. Detection of estrogen receptors ER-alpha and ER-beta in human ejaculated immature spermatozoa with excess residual cytoplasm. Reprod Biol Endocrinol. 2006; 4:36.16. Naraghi MA, Abolhasani F, Kashani I, Anarkooli IJ, Hemadi M, Azami A, Barbarestani M, Aitken RJ, Shokri S. The effects of swimming exercise and supraphysiological doses of nandrolone decanoate on the testis in adult male rats: a transmission electron microscope study. Folia Morphol (Warsz). 2010; 69:138–146.17. Bilinska B, Schmalz-Fraczek B, Kotula M, Carreau S. Photoperiod-dependent capability of androgen aromatization and the role of estrogens in the bank vole testis visualized by means of immunohistochemistry. Mol Cell Endocrinol. 2001; 178:189–198.18. Beltrame FL, Sasso-Cerri E. Vitamin B12-induced spermatogenesis recovery in cimetidine-treated rats: effect on the spermatogonia number and sperm concentration. Asian J Androl. 2017; 19:567–572.
Article19. Sasso-Cerri E, Miraglia SM. In situ demonstration of both TUNEL-labeled germ cell and Sertoli cell in the cimetidine-treated rats. Histol Histopathol. 2002; 17:411–417.20. Sasso-Cerri E, Cerri PS. Morphological evidences indicate that the interference of cimetidine on the peritubular components is responsible for detachment and apoptosis of Sertoli cells. Reprod Biol Endocrinol. 2008; 6:18.
Article21. Hooley RP, Paterson M, Brown P, Kerr K, Saunders PT. Intratesticular injection of adenoviral constructs results in Sertoli cell-specific gene expression and disruption of the seminiferous epithelium. Reproduction. 2009; 137:361–370.
Article22. Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev. 2004; 25:747–806.
Article23. Kianifard D, Sadrkhanlou RA, Hasanzadeh S. The ultrastructural changes of the sertoli and leydig cells following streptozotocin induced diabetes. Iran J Basic Med Sci. 2012; 15:623–635.24. Yamamoto Y, Sofikitis N, Mio Y, Loutradis D, Kaponis A, Miyagawa I. Morphometric and cytogenetic characteristics of testicular germ cells and Sertoli cell secretory function in men with non-mosaic Klinefelter's syndrome. Hum Reprod. 2002; 17:886–896.
Article25. Hutson JC. Altered biochemical responses by rat Sertoli cells and peritubular cells cultured under simulated diabetic conditions. Diabetologia. 1984; 26:155–158.
Article26. Tirado OM, Selva DM, Toran N, Suarez-Quian CA, Jansen M, McDonnell DP, Reventos J, Munell F. Increased expression of estrogen receptor beta in pachytene spermatocytes after short-term methoxyacetic acid administration. J Androl. 2004; 25:84–94.27. Simpkins JW, Yang SH, Sarkar SN, Pearce V. Estrogen actions on mitochondria: physiological and pathological implications. Mol Cell Endocrinol. 2008; 290:51–59.28. Gruber HE, Yamaguchi D, Ingram J, Leslie K, Huang W, Miller TA, Hanley EN Jr. Expression and localization of estrogen receptor-beta in annulus cells of the human intervertebral disc and the mitogenic effect of 17-beta-estradiol in vitro. BMC Musculoskelet Disord. 2002; 3:4.
Article29. Yang SH, Liu R, Perez EJ, Wen Y, Stevens SM Jr, Valencia T, Brun-Zinkernagel AM, Prokai L, Will Y, Dykens J, Koulen P, Simpkins JW. Mitochondrial localization of estrogen receptor beta. Proc Natl Acad Sci U S A. 2004; 101:4130–4135.30. Solakidi S, Psarra AM, Nikolaropoulos S, Sekeris CE. Estrogen receptors alpha and beta (ERalpha and ERbeta) and androgen receptor (AR) in human sperm: localization of ERbeta and AR in mitochondria of the midpiece. Hum Reprod. 2005; 20:3481–3487.31. Saunders PT, Millar MR, Macpherson S, Irvine DS, Groome NP, Evans LR, Sharpe RM, Scobie GA. ERbeta1 and the ERbeta2 splice variant (ERbetacx/beta2) are expressed in distinct cell populations in the adult human testis. J Clin Endocrinol Metab. 2002; 87:2706–2715.32. Selva DM, Tirado OM, Toran N, Suarez-Quian CA, Reventos J, Munell F. Estrogen receptor beta expression and apoptosis of spermatocytes of mice overexpressing a rat androgen-binding protein transgene. Biol Reprod. 2004; 71:1461–1468.33. Agarwal VR, Sinton CM, Liang C, Fisher C, German DC, Simpson ER. Upregulation of estrogen receptors in the forebrain of aromatase knockout (ArKO) mice. Mol Cell Endocrinol. 2000; 162:9–16.
Article34. de Ronde W, de Jong FH. Aromatase inhibitors in men: effects and therapeutic options. Reprod Biol Endocrinol. 2011; 9:93.
Article35. Sasso-Cerri E, Giovanoni M, Hayashi H, Miraglia SM. Morphological alterations and intratubular lipid inclusions as indicative of spermatogenic damage in cimetidine-treated rats. Arch Androl. 2001; 46:5–13.
Article36. Franca LR, Leal MC, Sasso-Cerri E, Vasconcelos A, Debeljuk L, Russell LD. Cimetidine (Tagamet) is a reproductive toxicant in male rats affecting peritubular cells. Biol Reprod. 2000; 63:1403–1412.37. Moreau F, Mittre H, Benhaim A, Bois C, Bertherat J, Carreau S, Reznik Y. Aromatase expression in the normal human adult adrenal and in adrenocortical tumors: biochemical, immunohistochemical, and molecular studies. Eur J Endocrinol. 2009; 160:93–99.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The effect of metformin on vitamin B12 level in pediatric patients
- Effect of Vitamin B12 on Nucleic Acid Metabolism in Lactobacillus leichmanii (Effect of Allicin on Nucleic Acid Metabolism)
- Effects of dietary supplementation of high-dose folic acid on biomarkers of methylating reaction in vitamin B12-deficient rats
- Vitamin B12 Deficiency after a Total Gastrectomy in Patients with Gastric Cancer
- The effects of exercise training and acute exercise duration on plasma folate and vitamin B12