Anat Cell Biol.
2018 Mar;51(1):41-51. 10.5115/acb.2018.51.1.41.
Morphine-alcohol treatment impairs cognitive functions and increases neuro-inflammatory responses in the medial prefrontal cortex of juvenile male rats
- Affiliations
-
- 1Department of Anatomy (Neuroscience Unit), Osun State University, Osogbo, Nigeria. adedayo.adekomi@uniosun.edu.ng
- 2Department of Anatomy, College of Medicine, Ekiti State University, Ado Ekiti, Nigeria.
- 3Department of Medical Biochemistry (Chemical Pathology Unit), Osun State University (Osogbo Campus), Osogbo, Nigeria.
- 4Department of Anatomy, Faculty of Basic Medical Science, University of Medical Sciences, Ondo, Nigeria.
- KMID: 2418394
- DOI: http://doi.org/10.5115/acb.2018.51.1.41
Abstract
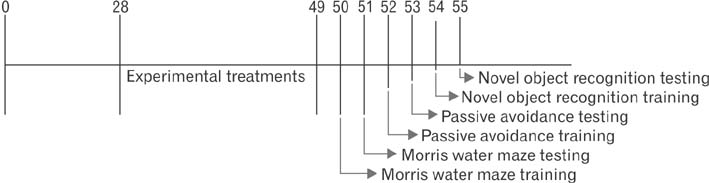
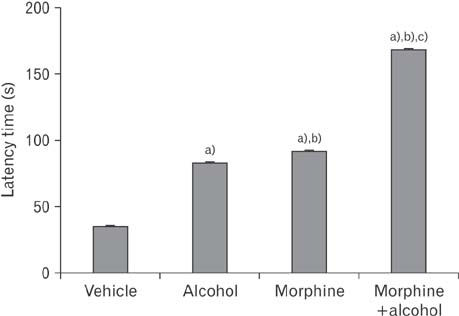
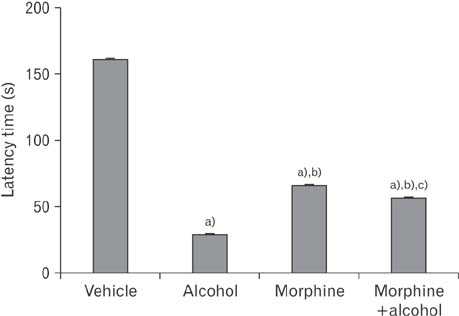
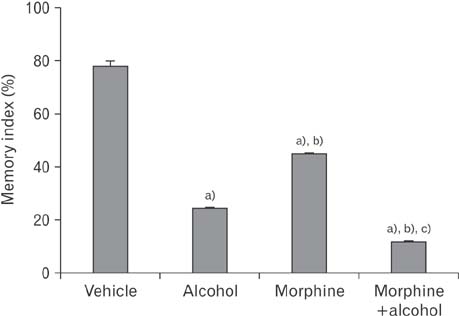
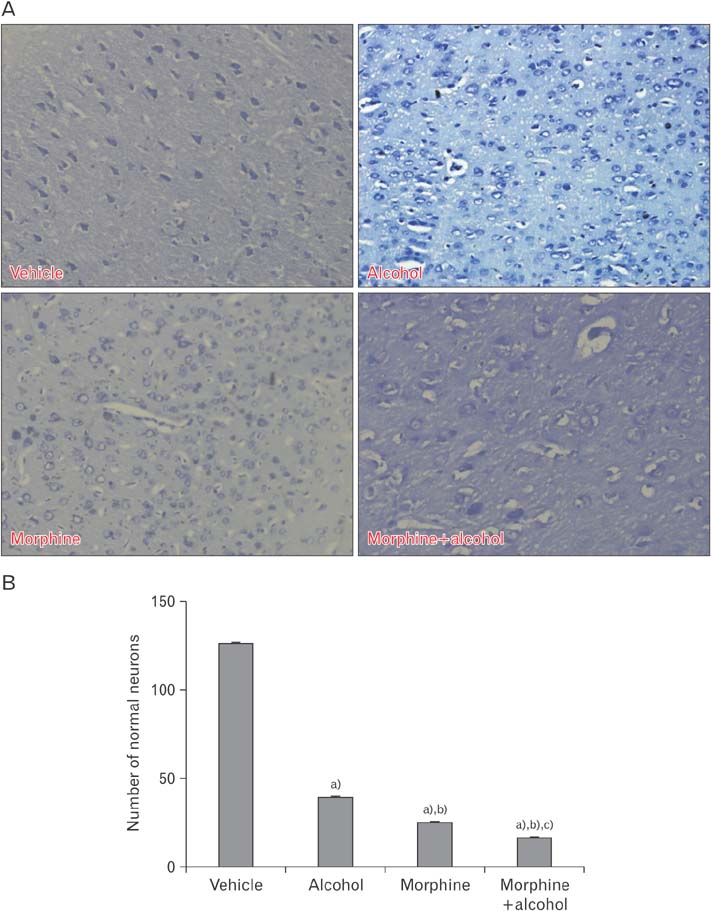
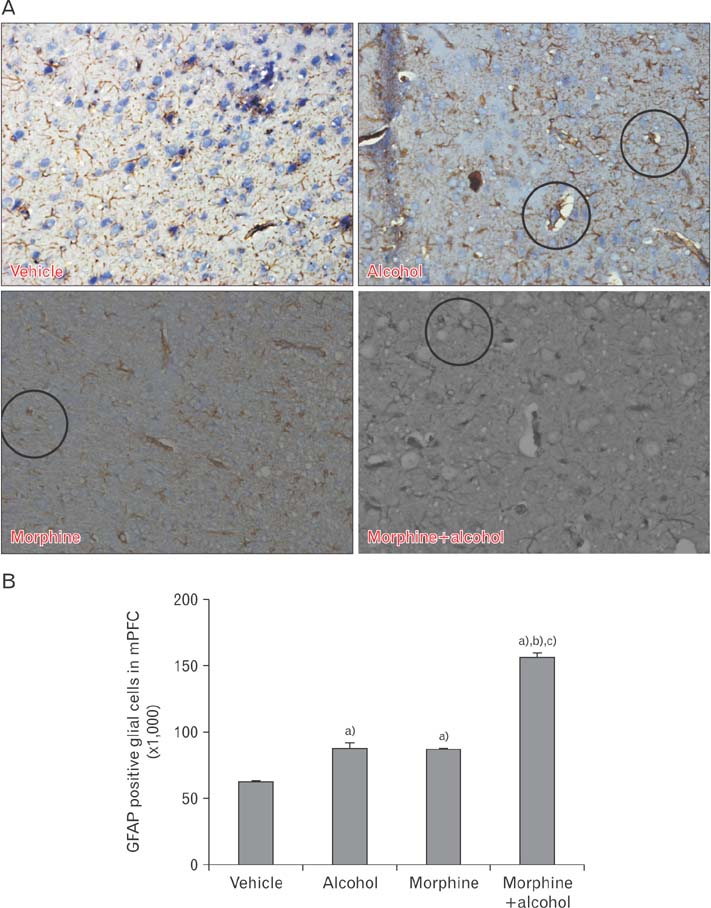
- In the developed and developing world, opioid consumption in combination with alcohol has become one of the substances abused. In this experiment, we examined the effects of alcohol, morphine, and morphine+alcohol combination on cognitive functions and neuroinflammatory responses in the medial prefrontal cortex (mPFC) of juvenile male rats. Alcohol (1.0 ml of 15% v/v ethanol twice daily, subcutaneously, 7 hours apart), morphine (0.5 ml/kg of 0.4 mg/kg morphine chlorate twice daily, subcutaneously, 7 hours apart), morphine+alcohol co-treatment (0.5 ml/kg of 0.4 mg/kg morphine chlorate+1.0 ml of 15% v/v ethanol twice daily, subcutaneously, 7 hours apart) were administered for 21 days. Treatment with morphine+alcohol significantly impairs cognition functions in the Morris water maze, passive avoidance, and novel object recognition tests, furthermore, the treatment significantly increased the quantitative count of astrocytic cells and also conferred marked neuronal cell death in the mPFC, which were studied by glial fibrillary acidic protein immunochemistry for astrocytes and Cresyl violet for Nissl's substance distribution in neurons respectively. These results suggest that alcohol, morphine, and morphine+alcohol co-treatment may trigger cognitive deficits and neuroinflammatory responses in the brain.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Curcumin supplementation shows modulatory influence on functional and morphological features of hippocampus in mice subjected to arsenic trioxide exposure
Kamakshi Mehta, Balpreet Kaur, Kamlesh Kumar Pandey, Saroj Kaler, Pushpa Dhar
Anat Cell Biol. 2020;53(3):355-365. doi: 10.5115/acb.18.169.
Reference
-
1. Kopnisky KL, Hyman SE. Molecular and cellular biology of addiction. In : Davis KL, Charney D, Coyle JT, Nemeroff C, editors. Neuropsychopharmacology: The Fifth Generation of Progress. Brentwood, TN: merican College of Neuropsychopharmacology;2002. p. 1367–1379.2. Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG. Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature. 2000; 408:720–723.3. Al-Hasani R, Bruchas MR. Molecular mechanisms of opioid receptor-dependent signaling and behavior. Anesthesiology. 2011; 115:1363–1381.
Article4. Martini L, Whistler JL. The role of mu opioid receptor desensitization and endocytosis in morphine tolerance and dependence. Curr Opin Neurobiol. 2007; 17:556–564.
Article5. Dumbili E. Changing patterns of alcohol consumption in Nigeria: an exploration of responsible factors and consequences. Med Soc Online. 2013; 7:20–33.6. Guerri C, Bazinet A, Riley EP. Foetal alcohol spectrum disorders and alterations in brain and behaviour. Alcohol Alcohol. 2009; 44:108–114.
Article7. Wilhelm CJ, Guizzetti M. Fetal alcohol spectrum disorders: an overview from the glia perspective. Front Integr Neurosci. 2016; 9:65.
Article8. Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann N Y Acad Sci. 2004; 1021:77–85.
Article9. Durston S, Davidson MC, Tottenham N, Galvan A, Spicer J, Fossella JA, Casey BJ. A shift from diffuse to focal cortical activity with development. Dev Sci. 2006; 9:1–8.
Article10. Counotte DS, Smit AB, Pattij T, Spijker S. Development of the motivational system during adolescence, and its sensitivity to disruption by nicotine. Dev Cogn Neurosci. 2011; 1:430–443.
Article11. Guerri C, Pascual M. Mechanisms involved in the neurotoxic, cognitive, and neurobehavioral effects of alcohol consumption during adolescence. Alcohol. 2010; 44:15–26.
Article12. Alfonso-Loeches S, Guerri C. Molecular and behavioral aspects of the actions of alcohol on the adult and developing brain. Crit Rev Clin Lab Sci. 2011; 48:19–47.
Article13. Crews FT, Braun CJ, Hoplight B, Switzer RC 3rd, Knapp DJ. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcohol Clin Exp Res. 2000; 24:1712–1723.
Article14. Pascual M, Blanco AM, Cauli O, Miñarro J, Guerri C. Intermittent ethanol exposure induces inflammatory brain damage and causes long-term behavioural alterations in adolescent rats. Eur J Neurosci. 2007; 25:541–550.
Article15. Attwood AS, Munafò MR. Effects of acute alcohol consumption and processing of emotion in faces: implications for understanding alcohol-related aggression. J Psychopharmacol. 2014; 28:719–732.
Article16. Kolb B, Mychasiuk R, Muhammad A, Li Y, Frost DO, Gibb R. Experience and the developing prefrontal cortex. Proc Natl Acad Sci U S A. 2012; 109:Suppl 2. 17186–17193.
Article17. Holroyd CB, Coles MG, Nieuwenhuis S. Medial prefrontal cortex and error potentials. Science. 2002; 296:1610–1611.
Article18. Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci. 2004; 8:539–546.
Article19. Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004; 306:443–447.
Article20. Bechara A, Damasio AR. The somatic marker hypothesis: a neural theory of economic decision. Games Econ Behav. 2005; 52:336–372.
Article21. Posner MI, Rothbart MK, Sheese BE, Tang Y. The anterior cingulate gyrus and the mechanism of self-regulation. Cogn Affect Behav Neurosci. 2007; 7:391–395.
Article22. Rushworth MF, Noonan MP, Boorman ED, Walton ME, Behrens TE. Frontal cortex and reward-guided learning and decision-making. Neuron. 2011; 70:1054–1069.
Article23. Euston DR, Gruber AJ, McNaughton BL. The role of medial prefrontal cortex in memory and decision making. Neuron. 2012; 76:1057–1070.
Article24. Shekarchizadeh H, Khami MR, Mohebbi SZ, Ekhtiari H, Virtanen JI. Oral health of drug abusers: a review of health effects and care. Iran J Public Health. 2013; 42:929–940.25. Park JH, Choi HY, Cho JH, Kim IH, Lee TK, Lee JC, Won MH, Chen BH, Shin BN, Ahn JH, Tae HJ, Choi JH, Chung JY, Lee CH, Cho JH, Kang IJ, Kim JD. Effects of chronic scopolamine treatment on cognitive impairments and myelin basic protein expression in the mouse hippocampus. J Mol Neurosci. 2016; 59:579–589.
Article26. Lee JC, Park JH, Ahn JH, Kim IH, Cho JH, Choi JH, Yoo KY, Lee CH, Hwang IK, Cho JH, Kwon YG, Kim YM, Kang IJ, Won MH. New GABAergic neurogenesis in the hippocampal CA1 region of a gerbil model of long-term survival after transient cerebral ischemic injury. Brain Pathol. 2016; 26:581–592.
Article27. Kim YH, Park JH. Vanillin and 4-hydroxybenzyl alcohol attenuate cognitive impairment and the reduction of cell proliferation and neuroblast differentiation in the dentate gyrus in a mouse model of scopolamine-induced amnesia. Anat Cell Biol. 2017; 50:143–151.
Article28. Adeniyi PA, Ishola AO, Laoye BJ, Olatunji BP, Bankole OO, Shallie PD, Ogundele OM. Neural and behavioural changes in male periadolescent mice after prolonged nicotine-MDMA treatment. Metab Brain Dis. 2016; 31:93–107.
Article29. Dribben WH, Creeley CE, Farber N. Low-level lead exposure triggers neuronal apoptosis in the developing mouse brain. Neurotoxicol Teratol. 2011; 33:473–480.
Article30. Paxinos G, Franklin K. Paxinos and Franklin's the mouse brain in stereotaxic coordinates. 4th ed. San Diego, CA: Academic Press;2012.31. Ma MX, Chen YM, He J, Zeng T, Wang JH. Effects of morphine and its withdrawal on Y-maze spatial recognition memory in mice. Neuroscience. 2007; 147:1059–1065.
Article32. Cippitelli A, Zook M, Bell L, Damadzic R, Eskay RL, Schwandt M, Heilig M. Reversibility of object recognition but not spatial memory impairment following binge-like alcohol exposure in rats. Neurobiol Learn Mem. 2010; 94:538–546.
Article33. Vetreno RP, Hall JM, Savage LM. Alcohol-related amnesia and dementia: animal models have revealed the contributions of different etiological factors on neuropathology, neurochemical dysfunction and cognitive impairment. Neurobiol Learn Mem. 2011; 96:596–608.
Article34. Swartzwelder HS, Acheson SK, Miller KM, Sexton HG, Liu W, Crews FT, Risher ML. Adolescent intermittent alcohol exposure: deficits in object recognition memory and forebrain cholinergic markers. PLoS One. 2015; 10:e0140042.
Article35. Nyberg F. Cognitive impairments in drug addicts. In : Gonzalez-Quevedo A, editor. Brain Damage: Bridging between Basic Research and Clinics. Rijeka: InTech;2012. p. 221–244.36. Hodgson PS, Neal JM, Pollock JE, Liu SS. The neurotoxicity of drugs given intrathecally (spinal). Anesth Analg. 1999; 88:797–809.
Article37. Boronat MA, García-Fuster MJ, García-Sevilla JA. Chronic morphine induces up-regulation of the pro-apoptotic Fas receptor and down-regulation of the anti-apoptotic Bcl-2 oncoprotein in rat brain. Br J Pharmacol. 2001; 134:1263–1270.
Article38. Mao J, Sung B, Ji RR, Lim G. Neuronal apoptosis associated with morphine tolerance: evidence for an opioid-induced neurotoxic mechanism. J Neurosci. 2002; 22:7650–7661.
Article39. Atici S, Cinel L, Cinel I, Doruk N, Aktekin M, Akca A, Camdeviren H, Oral U. Opioid neurotoxicity: comparison of morphine and tramadol in an experimental rat model. Int J Neurosci. 2004; 114:1001–1011.
Article40. Turchan-Cholewo J, Liu Y, Gartner S, Reid R, Jie C, Peng X, Chen KC, Chauhan A, Haughey N, Cutler R, Mattson MP, Pardo C, Conant K, Sacktor N, McArthur JC, Hauser KF, Gairola C, Nath A. Increased vulnerability of ApoE4 neurons to HIV proteins and opiates: protection by diosgenin and L-deprenyl. Neurobiol Dis. 2006; 23:109–119.
Article41. Zhang W, Hong JS, Kim HC, Zhang W, Block ML. Morphinan neuroprotection: new insight into the therapy of neurodegeneration. Crit Rev Neurobiol. 2004; 16:271–302.
Article42. Peart JN, Gross ER, Gross GJ. Opioid-induced preconditioning: recent advances and future perspectives. Vascul Pharmacol. 2005; 42:211–218.
Article43. Rambhia S, Mantione KJ, Stefano GB, Cadet P. Morphine modulation of the ubiquitin-proteasome complex is neuroprotective. Med Sci Monit. 2005; 11:BR386–BR396.44. Bekheet SH, Saker SA, Abdel-Kader AM, Younis AE. Histopathological and biochemical changes of morphine sulphate administration on the cerebellum of albino rats. Tissue Cell. 2010; 42:165–175.
Article45. Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010; 119:7–35.
Article46. Heller JP, Rusakov DA. Morphological plasticity of astroglia: understanding synaptic microenvironment. Glia. 2015; 63:2133–2151.
Article47. Block ML, Hong JS. Chronic microglial activation and progressive dopaminergic neurotoxicity. Biochem Soc Trans. 2007; 35(Pt 5):1127–1132.
Article48. Tremblay ME, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 2010; 8:e1000527.
Article49. Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT. Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med. 2011; 1:a006189.
Article50. Riancho J, Ruiz-Soto M, Villagrá NT, Berciano J, Berciano MT, Lafarga M. Compensatory motor neuron response to chromatolysis in the murine hSOD1(G93A) model of amyotrophic lateral sclerosis. Front Cell Neurosci. 2014; 8:346.
Article51. Liu LW, Lu J, Wang XH, Fu SK, Li Q, Lin FQ. Neuronal apoptosis in morphine addiction and its molecular mechanism. Int J Clin Exp Med. 2013; 6:540–545.52. Bajic D, Commons KG, Soriano SG. Morphine-enhanced apoptosis in selective brain regions of neonatal rats. Int J Dev Neurosci. 2013; 31:258–266.
Article53. Van den Oever MC, Spijker S, Smit AB, De Vries TJ. Prefrontal cortex plasticity mechanisms in drug seeking and relapse. Neurosci Biobehav Rev. 2010; 35:276–284.
Article54. Myers-Schulz B, Koenigs M. Functional anatomy of ventromedial prefrontal cortex: implications for mood and anxiety disorders. Mol Psychiatry. 2012; 17:132–141.
Article55. Rafati A, Noorafshan A, Torabi N. Stereological study of the effects of morphine consumption and abstinence on the number of the neurons and oligodendrocytes in medial prefrontal cortex of rats. Anat Cell Biol. 2013; 46:191–197.
Article56. Carlezon WA Jr, Nestler EJ. Elevated levels of GluR1 in the midbrain: a trigger for sensitization to drugs of abuse? Trends Neurosci. 2002; 25:610–615.
Article57. Mikkola JA, Janhunen S, Hyytiä P, Kiianmaa K, Ahtee L. Rotational behaviour in AA and ANA rats after repeated administration of morphine and cocaine. Pharmacol Biochem Behav. 2002; 73:697–702.
Article58. Ojanen S, Koistinen M, Bäckström P, Kankaanpää A, Tuomainen P, Hyytiä P, Kiianmaa K. Differential behavioural sensitization to intermittent morphine treatment in alcohol-preferring AA and alcohol-avoiding ANA rats: role of mesolimbic dopamine. Eur J Neurosci. 2003; 17:1655–1663.
Article59. Sanchis-Segura C, Pastor R, Aragon CM. Opposite effects of acute versus chronic naltrexone administration on ethanol-induced locomotion. Behav Brain Res. 2004; 153:61–67.
Article60. Kerns RT, Ravindranathan A, Hassan S, Cage MP, York T, Sikela JM, Williams RW, Miles MF. Ethanol-responsive brain region expression networks: implications for behavioral responses to acute ethanol in DBA/2J versus C57BL/6J mice. J Neurosci. 2005; 25:2255–2266.
Article61. Ojanen SP, Hyytia P, Kiianmaa K. Behavioral sensitization and voluntary ethanol drinking in alcohol-preferring AA rats exposed to different regimens of morphine treatment. Pharmacol Biochem Behav. 2005; 80:221–228.
Article62. Pastor R, Aragon CM. The role of opioid receptor subtypes in the development of behavioral sensitization to ethanol. Neuropsychopharmacology. 2006; 31:1489–1499.
Article63. Pastor R, Sanchis-Segura C, Aragon CM. Effect of selective antagonism of mu(1)-, mu(1/2)-, mu(3)-, and delta-opioid receptors on the locomotor-stimulating actions of ethanol. Drug Alcohol Depend. 2005; 78:289–295.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Three Musketeers in the Medial Prefrontal Cortex: Subregion-specific Structural and Functional Plasticity Underlying Fear Memory Stages
- Stereological study of the effects of morphine consumption and abstinence on the number of the neurons and oligodendrocytes in medial prefrontal cortex of rats
- Functions of the prefrontal cortex in the human brain
- Inactivation of the Medial Prefrontal Cortex Interferes with the Expression But Not the Acquisition of Differential Fear Conditioning in Rats
- Differential Changes in Neural Activity of Prefrontal Cortical Neurons of Behaving Rats by Clozapine Administration