Clin Exp Vaccine Res.
2018 Jul;7(2):129-138. 10.7774/cevr.2018.7.2.129.
Development of dual reporter imaging system for Francisella tularensis to monitor the spatio-temporal pathogenesis and vaccine efficacy
- Affiliations
-
- 1Department of Nuclear Medicine, Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea. hwyoun@snu.ac.kr
- 2Department of Microbiology, Yonsei University College of Medicine, Seoul, Korea.
- 3International Vaccine Institute, Seoul, Korea.
- 4Interpark Bio-Convergence Center, I-Market-Korea, Seoul, Korea. kevin.hong@imarketkorea.com
- 5Cancer Imaging Center, Seoul National University Hospital, Seoul, Korea.
- KMID: 2418091
- DOI: http://doi.org/10.7774/cevr.2018.7.2.129
Abstract
- PURPOSE
Study on the pathogen and the pathogen-related disease require the information at both cellular and organism level. However, lack of appropriate high-quality antibodies and the difference between the experimental animal models make it difficult to analyze in vivo mechanism of pathogen-related diseases. For more reliable research on the infection and immune-response of pathogen-related diseases, accurate analysis is essential to provide spatiotemporal information of pathogens and immune activity to avoid false-positive or mis-interpretations. In this regards, we have developed a method for tracking Francisella tularensis in the animal model without using the specific antibodies for the F. tularensis.
MATERIALS AND METHODS
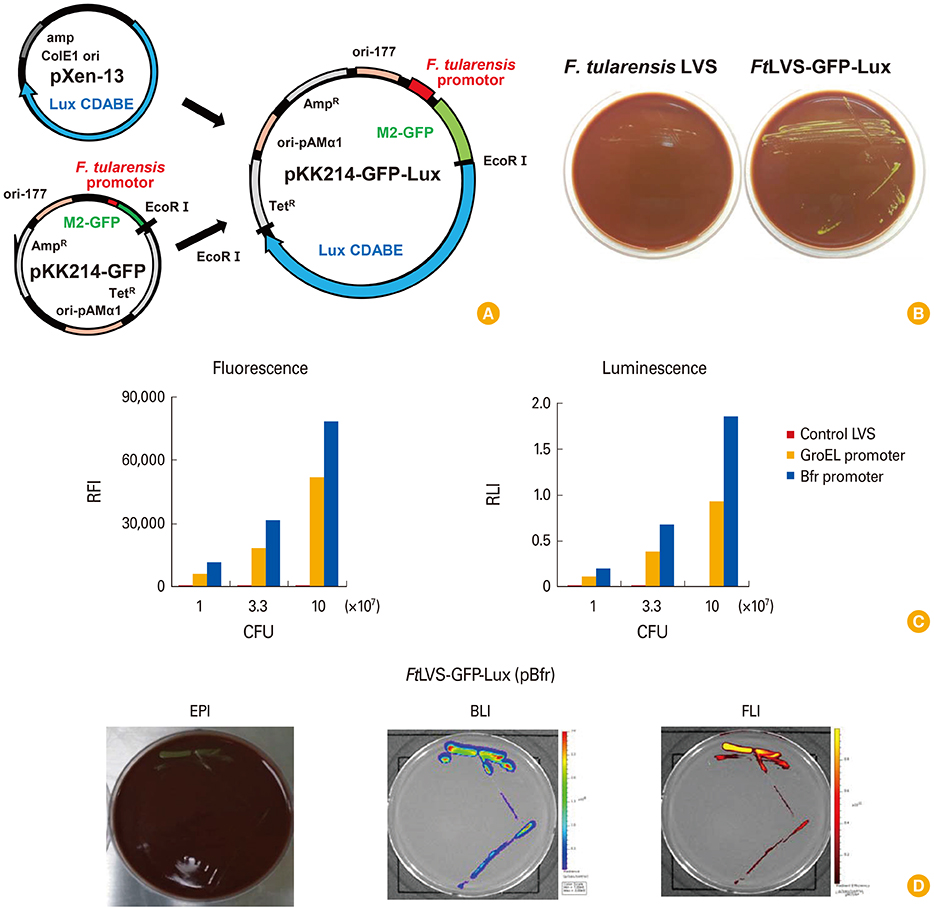
A dual reporter plasmid using GFP-Lux with putative bacterioferritin promoter (pBfr) was constructed and transformed to F. tularensis live vaccine strain to generate F. tularensis LVS (FtLVS)-GFP-Lux for both fluorescence and bioluminescence imaging. For vaccination to F. tularensis infection, FtLVS and lipopolysaccharide (LPS) from FtLVS were used.
RESULTS
We visualized the bacterial replication of F. tularensis in the cells using fluorescence and bioluminescence imaging, and traced the spatio-temporal process of F. tularensis pathogenesis in mice. Vaccination with LPS purified from FtLVS greatly reduced the bacterial replication of FtLVS in animal model, and the effect of vaccination was also successfully monitored with in vivo imaging.
CONCLUSION
We successfully established dual reporter labeled F. tularensis for cellular and whole body imaging. Our simple and integrated imaging analysis system would provide useful information for in vivo analysis of F. tularensis infection as well as in vitro experiments, which have not been fully explained yet with various technical problems.
Keyword
MeSH Terms
Figure
Reference
-
1. Meighen EA. Molecular biology of bacterial bioluminescence. Microbiol Rev. 1991; 55:123–142.
Article2. Golovliov I, Baranov V, Krocova Z, Kovarova H, Sjostedt A. An attenuated strain of the facultative intracellular bacterium Francisella tularensis can escape the phagosome of monocytic cells. Infect Immun. 2003; 71:5940–5950.
Article3. Bina XR, Miller MA, Bina JE. Construction of a bioluminescence reporter plasmid for Francisella tularensis. Plasmid. 2010; 64:156–161.
Article4. Giorno R, Mallozzi M, Bozue J, et al. Localization and assembly of proteins comprising the outer structures of the Bacillus anthracis spore. Microbiology. 2009; 155:1133–1145.
Article5. Glomski IJ, Piris-Gimenez A, Huerre M, Mock M, Goossens PL. Primary involvement of pharynx and peyer's patch in inhalational and intestinal anthrax. PLoS Pathog. 2007; 3:e76.
Article6. Louie M, Read S, Simor AE, et al. Application of multiplex PCR for detection of non-O157 verocytotoxin-producing Escherichia coli in bloody stools: identification of serogroups O26 and O111. J Clin Microbiol. 1998; 36:3375–3377.
Article7. Rajwa B, Dundar MM, Akova F, et al. Discovering the unknown: detection of emerging pathogens using a label-free light-scattering system. Cytometry A. 2010; 77:1103–1112.
Article8. Shachaf CM, Kopelman AM, Arvanitis C, et al. MYC inactivation uncovers pluripotent differentiation and tumour dormancy in hepatocellular cancer. Nature. 2004; 431:1112–1117.
Article9. Burton JB, Johnson M, Sato M, et al. Adenovirus-mediated gene expression imaging to directly detect sentinel lymph node metastasis of prostate cancer. Nat Med. 2008; 14:882–888.
Article10. Nasu M, Carbone M, Gaudino G, et al. Ranpirnase interferes with NF-kappaB pathway and MMP9 activity, inhibiting malignant mesothelioma cell invasiveness and xenograft growth. Genes Cancer. 2011; 2:576–584.
Article11. Oyston PC, Sjostedt A, Titball RW. Tularaemia: bioterrorism defence renews interest in Francisella tularensis. Nat Rev Microbiol. 2004; 2:967–978.
Article12. Pechous RD, McCarthy TR, Zahrt TC. Working toward the future: insights into Francisella tularensis pathogenesis and vaccine development. Microbiol Mol Biol Rev. 2009; 73:684–711.
Article13. Hong KJ, Park PG, Seo SH, Rhie GE, Hwang KJ. Current status of vaccine development for tularemia preparedness. Clin Exp Vaccine Res. 2013; 2:34–39.
Article14. Green M, Choules G, Rogers D, Titball RW. Efficacy of the live attenuated Francisella tularensis vaccine (LVS) in a murine model of disease. Vaccine. 2005; 23:2680–2686.
Article15. Wang X, Zhang X, Zhou D, Yang R. Live-attenuated Yersinia pestis vaccines. Expert Rev Vaccines. 2013; 12:677–686.16. Herwig L, Rice AJ, Bedbrook CN, et al. Directed evolution of a bright near-infrared fluorescent rhodopsin using a synthetic chromophore. Cell Chem Biol. 2017; 24:415–425.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current status of vaccine development for tularemia preparedness
- The first pediatric case of tularemia in Korea: manifested with pneumonia and possible infective endocarditis
- A Case of Tularemia Caused by Francisella Tularensis
- Imaging Gene Expression
- Cytokine response in Balb/c mice infected with Francisella tularensis LVS and the Pohang isolate