Cancer Res Treat.
2018 Jul;50(3):691-700. 10.4143/crt.2017.280.
Crizotinib versus Chemotherapy in Asian Patients with ALK-Positive Advanced Non-small Cell Lung Cancer
- Affiliations
-
- 1Thoracic Oncology Center, The Cancer Institute Hospital of JFCR, Tokyo, Japan. mnishio@jfcr.or.jp
- 2Seoul National University Hospital, Seoul, Korea.
- 3Guangdong Lung Cancer Institute, Guangdong, China.
- 4Kindai University, Osaka, Japan.
- 5Peter MacCallum Cancer Centre, Melbourne, Australia.
- 6Massachusetts General Hospital, Boston, MA, USA.
- 7Pfizer Oncology, Tokyo, Japan.
- 8Pfizer Oncology, Milan, Italy.
- 9Pfizer Oncology, La Jolla, CA, USA.
- 10State Key Laboratory of South China, Hong Kong Cancer Institute and The Chinese University of Hong Kong, Shatin, China.
- KMID: 2417858
- DOI: http://doi.org/10.4143/crt.2017.280
Abstract
- PURPOSE
Crizotinib has demonstrated superior progression-free survival (PFS) and objective response rates (ORRs) versus chemotherapy in previously treated and untreated patients with anaplastic lymphoma kinase (ALK)-positive advanced non-small cell lung cancer (NSCLC). We report the safety and efficacy of crizotinib in Asian subpopulations of two global phase III trials.
MATERIALS AND METHODS
This analysis evaluated previously treated and untreated patients in two randomized, open-label phase III trials of crizotinib versus chemotherapy in ALK-positive advanced NSCLC in second-line (PROFILE 1007) and first-line settings (PROFILE 1014). Efficacy and safety were analyzed by race in the intention-to-treat and "as-treated" populations for efficacy and safety endpoints, respectively.
RESULTS
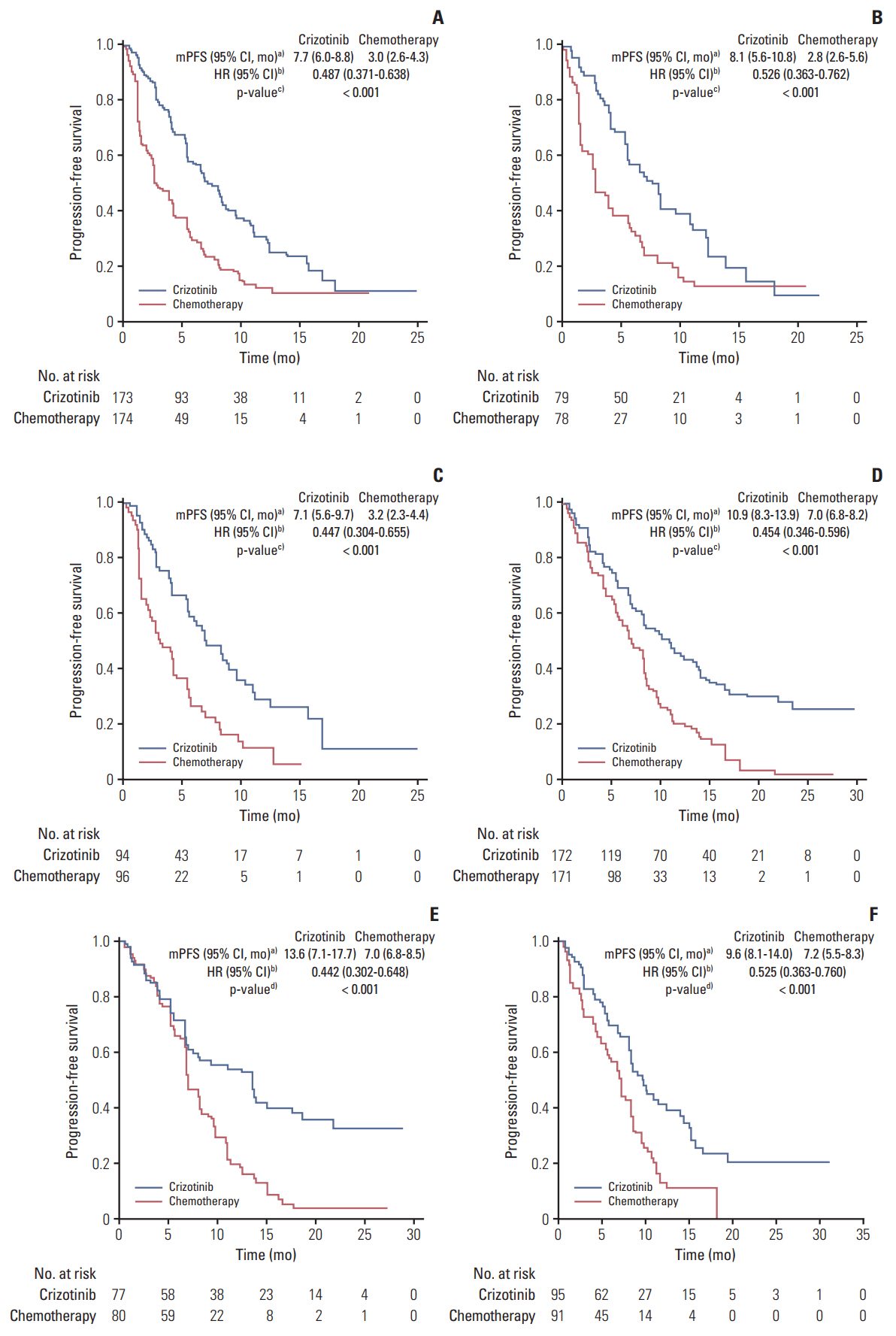
In previously treated (n=157) and untreated (n=157) Asian patients, PFS was statistically significantly longer with crizotinib versus chemotherapy (hazard ratio for PFS, 0.526; 95% confidence interval, 0.363 to 0.762; p < 0.001 and hazard ratio, 0.442; 95% confidence interval, 0.302 to 0.648; p < 0.001, respectively). Similar antitumor activity was seen in the non-Asian and overall populations. ORRs were statistically significantly higher with crizotinib versus chemotherapy in both Asian and non-Asian previously treated and untreated patients (p < 0.05). The most common treatment-emergent adverse events (any grade)with crizotinib were vision disorder, diarrhea, and nausea, which were observed at a comparable incidence across Asian and non-Asian populations, irrespective of previous treatment status. Most adverse events were mild to moderate in severity.
CONCLUSION
These data, currently the only analysis showing Asian and non-Asian populations in the same study, support the efficacy and safety of crizotinib in Asian patients with previously treated or untreated ALK-positive advanced NSCLC.
MeSH Terms
Figure
Cited by 1 articles
-
Efficacy and Safety of Ceritinib 450 mg/day with Food and 750 mg/day in Fasted State in Treatment-Naïve Patients with
ALK + Non–Small Cell Lung Cancer: Results from the ASCEND-8 Asian Subgroup Analysis
Byoung Chul Cho, Dong-Wan Kim, Ullas Batra, Keunchil Park, Sang-We Kim, Cheng-Ta Yang, Pei-Jye Voon, Virote Sriuranpong, K. Govind Babu, Khalid Amin, Yingbo Wang, Paramita Sen, Khemaies Slimane, Sarayut Geater
Cancer Res Treat. 2023;55(1):83-93. doi: 10.4143/crt.2021.1571.
Reference
-
References
1. Grande E, Bolos MV, Arriola E. Targeting oncogenic ALK: a promising strategy for cancer treatment. Mol Cancer Ther. 2011; 10:569–79.
Article2. Gridelli C, Peters S, Sgambato A, Casaluce F, Adjei AA, Ciardiello F. ALK inhibitors in the treatment of advanced NSCLC. Cancer Treat Rev. 2014; 40:300–6.
Article3. Shaw AT, Yeap BY, Mino-Kenudson M, Digumarthy SR, Costa DB, Heist RS, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009; 27:4247–53.
Article4. Shaw AT, Engelman JA. ALK in lung cancer: past, present, and future. J Clin Oncol. 2013; 31:1105–11.5. Camidge DR, Bang YJ, Kwak EL, Iafrate AJ, Varella-Garcia M, Fox SB, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol. 2012; 13:1011–9.
Article6. Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010; 363:1693–703.7. Kim D, Ahn M, Yang P, Liu X, De Pas T, Crino L, et al. Updated results of a global phase II study with crizotinib in advanced ALK-positive non-small cell lung cancer. Ann Oncol. 2012; 23:402.8. Shaw AT, Kim DW, Nakagawa K, Seto T, Crino L, Ahn MJ, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013; 368:2385–94.
Article9. Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014; 371:2167–77.
Article10. Lu S, Mok T, Lu Y, Zhou J, Shi Y, Sriuranpong V, et al. Phase 3 study of first-line crizotinib vs pemetrexed-cisplatin/carboplatin (PCC) in East Asian patients (pts) with ALK+ advanced non-squamous non-small cell lung cancer (NSCLC). J Clin Oncol. 2016; 34(15 Suppl):Abstr.
Article11. Ou SH, Salgia R, Clark J, Kwak EL, Camidge DR, Maki R, et al. Comparison of crizotinib (PF-02341066) pharmacokinetics between Asian and non-Asian patients with advanced malignancies. J Thorac Oncol. 2010; 5:S382.12. Park K, Kim JH, Cho EK, Kang JH, Shih JY, Zimmermann AH, et al. East Asian subgroup analysis of a randomized, double-blind, phase 3 study of docetaxel and ramucirumab versus docetaxel and placebo in the treatment of stage IV non-small cell lung cancer following disease progression after one prior platinum-based therapy (REVEL). Cancer Res Treat. 2016; 48:1177–86.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Successful Treatment of Crizotinib-Associated Severe Hepatotoxicity in Anaplastic Lymphoma Kinase-Positive Non-Small Cell Lung Cancer
- Clinical factors affecting progression-free survival with crizotinib in ALK-positive non-small cell lung cancer
- Crizotinib in Combination with Everolimus Synergistically Inhibits Proliferation of Anaplastic Lymphoma Kinase‒Positive Anaplastic Large Cell Lymphoma
- Uterine Cervix Metastasis in Lung Adenocarcinoma with Anaplastic Lymphoma Kinase Rearrangement
- Bilateral Ovarian Metastases from ALK Rearranged Non-Small Cell Lung Cancer