Yonsei Med J.
2018 Aug;59(6):727-735. 10.3349/ymj.2018.59.6.727.
Synergistic Anti-Cancer Effects of AKT and SRC Inhibition in Human Pancreatic Cancer Cells
- Affiliations
-
- 1Department of Physiology, School of Medicine, CHA University, Seongnam, Korea. leedh@cha.ac.kr
- 2Department of Preventive Medicine, School of Medicine, CHA University, Seongnam, Korea.
- KMID: 2415531
- DOI: http://doi.org/10.3349/ymj.2018.59.6.727
Abstract
- PURPOSE
To investigate the effect of combined inhibition of protein kinase B (AKT) and SRC on the growth and metastatic potential of human pancreatic cancer cells.
MATERIALS AND METHODS
AKT and SRC were inhibited using 10-DEBC and PP2, respectively. The expression of their messenger RNAs were down-regulated by specific small interfering RNA (siRNA). Changes in pancreatic cancer cell growth and metastatic potential were determined using a cell viability assay and a xenotransplant model of pancreatic cancer, as well as cell migration and invasion assays. Signal proteins were analyzed by Western blot.
RESULTS
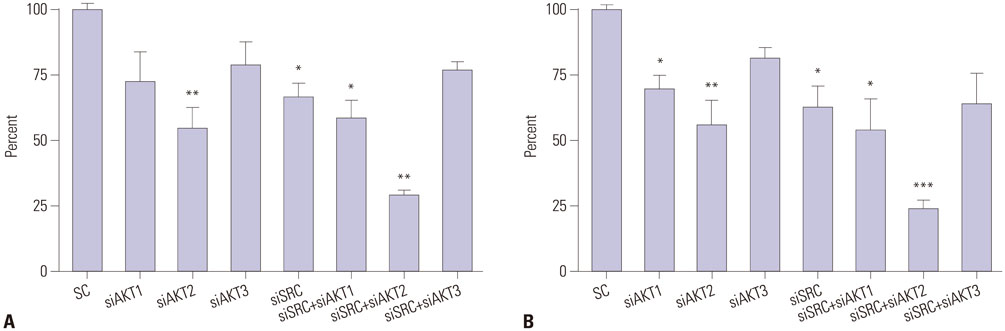
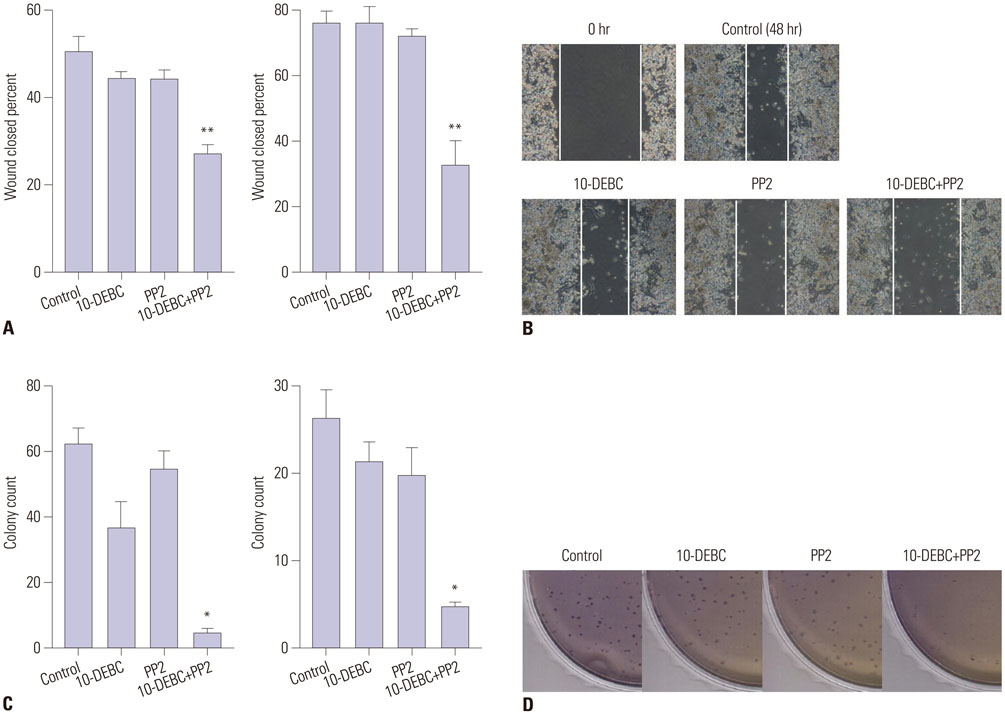
The inhibitors 10-DEBC and PP2 suppressed cell proliferation in a dose-dependent fashion in pancreatic cancer cell lines MIA PaCa-2 and PANC-1. The simultaneous inhibition of AKT and SRC at low concentrations resulted in a significant suppression of cell proliferation. Knockdown of AKT2 and SRC using siRNAs also significantly decreased cell proliferation. In a pancreatic cancer model, combined treatment with 10-DEBC and PP2 also significantly suppressed the growth of pancreatic cancer. Application of 10-DEBC with PP2 significantly reduced the metastatic potential of pancreatic cancer cells by inhibiting migration and invasion. The combined inhibition suppressed the phosphorylation of mTOR and ERK in pancreatic cancer cells.
CONCLUSION
Combined targeting of AKT and SRC resulted in a synergistic efficacy against human pancreatic cancer growth and metastasis.
Keyword
MeSH Terms
Figure
Reference
-
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015; 65:5–29.
Article2. Hidalgo M. Pancreatic cancer. N Engl J Med. 2010; 362:1605–1617.
Article3. Nieto J, Grossbard ML, Kozuch P. Metastatic pancreatic cancer 2008: is the glass less empty? Oncologist. 2008; 13:562–576.
Article4. Squadroni M, Fazio N. Chemotherapy in pancreatic adenocarcinoma. Eur Rev Med Pharmacol Sci. 2010; 14:386–394.5. Stathis A, Moore MJ. Advanced pancreatic carcinoma: current treatment and future challenges. Nat Rev Clin Oncol. 2010; 7:163–172.
Article6. Je DW, O YM, Ji YG, Cho Y, Lee DH. The inhibition of SRC family kinase suppresses pancreatic cancer cell proliferation, migration, and invasion. Pancreas. 2014; 43:768–776.
Article7. Wheeler DL, Iida M, Dunn EF. The role of Src in solid tumors. Oncologist. 2009; 14:667–678.
Article8. Choi JH, Ji YG, Lee DH. Uridine triphosphate increases proliferation of human cancerous pancreatic duct epithelial cells by activating P2Y2 receptor. Pancreas. 2013; 42:680–686.
Article9. Trevino JG, Summy JM, Lesslie DP, Parikh NU, Hong DS, Lee FY, et al. Inhibition of SRC expression and activity inhibits tumor progression and metastasis of human pancreatic adenocarcinoma cells in an orthotopic nude mouse model. Am J Pathol. 2006; 168:962–972.
Article10. Bjorge JD, Pang AS, Funnell M, Chen KY, Diaz R, Magliocco AM, et al. Simultaneous siRNA targeting of Src and downstream signaling molecules inhibit tumor formation and metastasis of a human model breast cancer cell line. PLoS One. 2011; 6:e19309.
Article11. Chang YM, Bai L, Liu S, Yang JC, Kung HJ, Evans CP. Src family kinase oncogenic potential and pathways in prostate cancer as revealed by AZD0530. Oncogene. 2008; 27:6365–6375.
Article12. Yezhelyev MV, Koehl G, Guba M, Brabletz T, Jauch KW, Ryan A, et al. Inhibition of SRC tyrosine kinase as treatment for human pancreatic cancer growing orthotopically in nude mice. Clin Cancer Res. 2004; 10:8028–8036.
Article13. Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009; 9:550–562.
Article14. Altomare DA, Testa JR. Perturbations of the AKT signaling pathway in human cancer. Oncogene. 2005; 24:7455–7464.
Article15. Roy SK, Srivastava RK, Shankar S. Inhibition of PI3K/AKT and MAPK/ERK pathways causes activation of FOXO transcription factor, leading to cell cycle arrest and apoptosis in pancreatic cancer. J Mol Signal. 2010; 5:10.
Article16. Chen KF, Yeh PY, Yeh KH, Lu YS, Huang SY, Cheng AL. Down-regulation of phospho-Akt is a major molecular determinant of bortezomib-induced apoptosis in hepatocellular carcinoma cells. Cancer Res. 2008; 68:6698–6707.
Article17. Gallia GL, Tyler BM, Hann CL, Siu IM, Giranda VL, Vescovi AL, et al. Inhibition of Akt inhibits growth of glioblastoma and glioblastoma stem-like cells. Mol Cancer Ther. 2009; 8:386–393.
Article18. Yu JH, Kim H. Role of janus kinase/signal transducers and activators of transcription in the pathogenesis of pancreatitis and pancreatic cancer. Gut Liver. 2012; 6:417–422.
Article19. Furukawa T. Molecular targeting therapy for pancreatic cancer: current knowledge and perspectives from bench to bedside. J Gastroenterol. 2008; 43:905–911.
Article20. Lee DH, Chung K, Song JA, Kim TH, Kang H, Huh JH, et al. Proteomic identification of paclitaxel-resistance associated hnRNP A2 and GDI 2 proteins in human ovarian cancer cells. J Proteome Res. 2010; 9:5668–5676.
Article21. Choi JH, Ji YG, Ko JJ, Cho HJ, Lee DH. Activating P2X7 receptors increases proliferation of human pancreatic cancer cells via ERK1/2 and JNK. Pancreas. 2018; 47:643–651.
Article22. Renouf DJ, Moore MJ, Hedley D, Gill S, Jonker D, Chen E, et al. A phase I/II study of the Src inhibitor saracatinib (AZD0530) in combination with gemcitabine in advanced pancreatic cancer. Invest New Drugs. 2012; 30:779–786.
Article23. Trevino JG, Summy JM, Gray MJ, Nilsson MB, Lesslie DP, Baker CH, et al. Expression and activity of SRC regulate interleukin-8 expression in pancreatic adenocarcinoma cells: implications for angiogenesis. Cancer Res. 2005; 65:7214–7222.
Article24. Summy JM, Trevino JG, Baker CH, Gallick GE. c-Src regulates constitutive and EGF-mediated VEGF expression in pancreatic tumor cells through activation of phosphatidyl inositol-3 kinase and p38 MAPK. Pancreas. 2005; 31:263–274.
Article25. Duxbury MS, Ito H, Zinner MJ, Ashley SW, Whang EE. siRNA directed against c-Src enhances pancreatic adenocarcinoma cell gemcitabine chemosensitivity. J Am Coll Surg. 2004; 198:953–959.
Article26. Tanno S, Tanno S, Mitsuuchi Y, Altomare DA, Xiao GH, Testa JR. AKT activation up-regulates insulin-like growth factor I receptor expression and promotes invasiveness of human pancreatic cancer cells. Cancer Res. 2001; 61:589–593.27. Ito H, Gardner-Thorpe J, Zinner MJ, Ashley SW, Whang EE. Inhibition of tyrosine kinase Src suppresses pancreatic cancer invasiveness. Surgery. 2003; 134:221–226.
Article28. Rajeshkumar NV, Tan AC, De Oliveira E, Womack C, Wombwell H, Morgan S, et al. Antitumor effects and biomarkers of activity of AZD0530, a Src inhibitor, in pancreatic cancer. Clin Cancer Res. 2009; 15:4138–4146.
Article29. Messersmith WA, Rajeshkumar NV, Tan AC, Wang XF, Diesl V, Choe SE, et al. Efficacy and pharmacodynamic effects of bosutinib (SKI-606), a Src/Abl inhibitor, in freshly generated human pancreas cancer xenografts. Mol Cancer Ther. 2009; 8:1484–1493.
Article30. Irby RB, Yeatman TJ. Role of Src expression and activation in human cancer. Oncogene. 2000; 19:5636–5642.
Article31. Duxbury MS, Ito H, Benoit E, Zinner MJ, Ashley SW, Whang EE. Overexpression of CEACAM6 promotes insulin-like growth factor I-induced pancreatic adenocarcinoma cellular invasiveness. Oncogene. 2004; 23:5834–5842.
Article32. Jaganathan S, Yue P, Turkson J. Enhanced sensitivity of pancreatic cancer cells to concurrent inhibition of aberrant signal transducer and activator of transcription 3 and epidermal growth factor receptor or Src. J Pharmacol Exp Ther. 2010; 333:373–381.
Article33. Nagaraj NS, Washington MK, Merchant NB. Combined blockade of Src kinase and epidermal growth factor receptor with gemcitabine overcomes STAT3-mediated resistance of inhibition of pancreatic tumor growth. Clin Cancer Res. 2011; 17:483–493.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Synergistic Effect of Sulindac and Simvastatin on Apoptosis in Lung Cancer A549 Cells through AKT-Dependent Downregulation of Survivin
- Dasatinib induces apoptosis and autophagy by suppressing the PI3K/Akt/mTOR pathway in bladder cancer cells
- Sunitinib Malate Synergistically Potentiates Anti-Tumor Effect of Gemcitabine in Human Bladder Cancer Cells
- Antitumor Effects of Fucoidan on Human Colon Cancer Cells via Activation of Akt Signaling
- Pan-Pim Kinase Inhibitor AZD1208 Suppresses Tumor Growth and Synergistically Interacts with Akt Inhibition in Gastric Cancer Cells