J Gastric Cancer.
2018 Jun;18(2):134-141. 10.5230/jgc.2018.18.e15.
Pancreatic Compression during Lymph Node Dissection in Laparoscopic Gastrectomy: Possible Cause of Pancreatic Leakage
- Affiliations
-
- 1Department of Gastroenterological Surgery, Cancer Institute Hospital of the Japanese Foundation for Cancer Research, Tokyo, Japan. naoki.hiki@jfcr.or.jp
- 2Laboratory of Chemistry and Biology, Graduate School of Pharmaceutical Sciences, The University of Tokyo, Tokyo, Japan.
- 3Laboratory of Chemical Biology and Molecular Imaging, Graduate School of Medicine, The University of Tokyo, Tokyo, Japan.
- 4Precursory Research for Embryonic Science and Technology (PRESTO), Japan Science and Technology Agency, Saitama, Japan.
- 5Core Research for Evolutional Science and Technology (CREST), Japan Agency for Medical Research and Development, Tokyo, Japan.
- 6Division of Carcinogenesis, Cancer Institute, Japanese Foundation for Cancer Research, Tokyo, Japan.
- KMID: 2414533
- DOI: http://doi.org/10.5230/jgc.2018.18.e15
Abstract
- PURPOSE
Postoperative pancreatic fistula is a serious and fatal complication of gastrectomy for gastric cancer. Blunt trauma to the parenchyma of the pancreas can result from an assistant's forceps compressing and retracting the pancreas, which in turn may result in pancreatic juice leakage. However, no published studies have focused on blunt trauma to the pancreas during laparoscopic surgery. Our aim was to investigate the relationship between compression of the pancreas and pancreatic juice leakage in a swine model.
MATERIALS AND METHODS
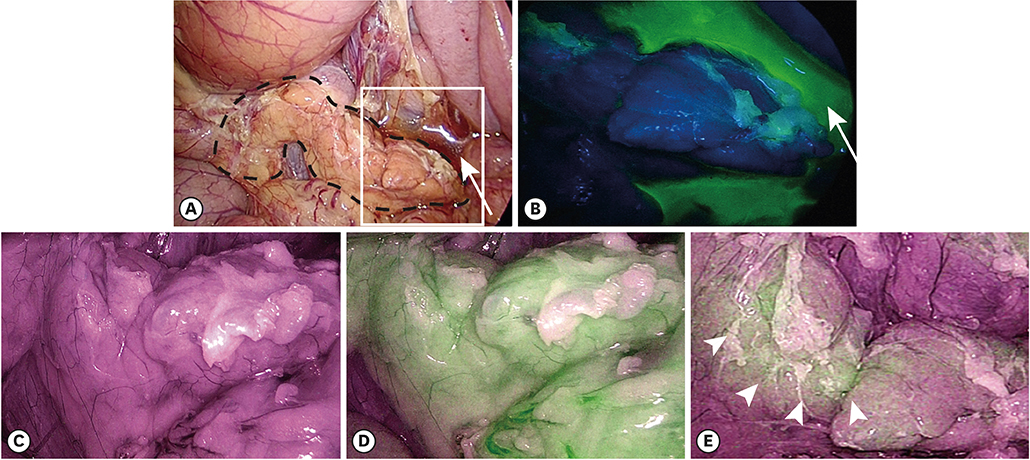
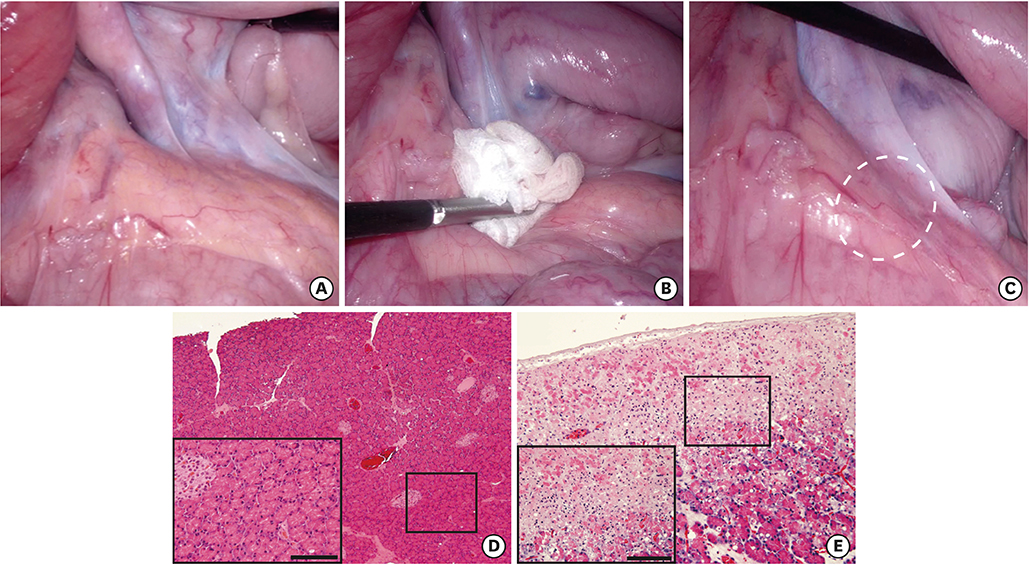
Three female pigs were used in this study. The pancreas was gently compressed dorsally for 15 minutes laparoscopically with gauze grasped with forceps. Pancreatic juice leakage was visualized by fluorescence imaging after topical administration of chymotrypsin-activatable fluorophore in real time. Amylase concentrations in ascites collected at specified times was measured. In addition, pancreatic tissue was fixed with formalin, and the histology of the compressed sites was evaluated.
RESULTS
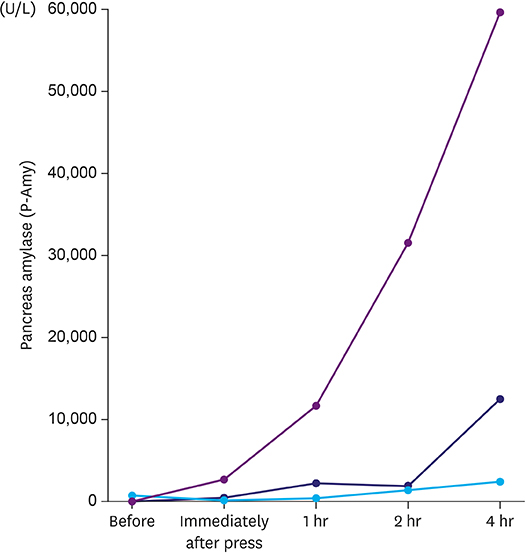
Fluorescence imaging enabled visualization of pancreatic juice leaking into ascites around the pancreas. Median concentrations of pancreatic amylase in ascites increased from 46 U/L preoperatively to 12,509 U/L 4 hours after compression. Histological examination of tissues obtained 4 hours after compression revealed necrotic pancreatic acinar cells extending from the surface to deep within the pancreas and infiltration of inflammatory cells.
CONCLUSIONS
Pancreatic compression by the assistant's forceps can contribute to pancreatic juice leakage. These findings will help to improve the procedure for lymph node dissection around the pancreas during laparoscopic gastrectomy.
Keyword
MeSH Terms
Figure
Reference
-
1. Hosono S, Arimoto Y, Ohtani H, Kanamiya Y. Meta-analysis of short-term outcomes after laparoscopy-assisted distal gastrectomy. World J Gastroenterol. 2006; 12:7676–7683.
Article2. Viñuela EF, Gonen M, Brennan MF, Coit DG, Strong VE. Laparoscopic versus open distal gastrectomy for gastric cancer: a meta-analysis of randomized controlled trials and high-quality nonrandomized studies. Ann Surg. 2012; 255:446–456.3. Yakoub D, Athanasiou T, Tekkis P, Hanna GB. Laparoscopic assisted distal gastrectomy for early gastric cancer: is it an alternative to the open approach? Surg Oncol. 2009; 18:322–333.
Article4. Zeng YK, Yang ZL, Peng JS, Lin HS, Cai L. Laparoscopy-assisted versus open distal gastrectomy for early gastric cancer: evidence from randomized and nonrandomized clinical trials. Ann Surg. 2012; 256:39–52.5. Irino T, Hiki N, Ohashi M, Nunobe S, Sano T, Yamaguchi T. The Hit and Away technique: optimal usage of the ultrasonic scalpel in laparoscopic gastrectomy. Surg Endosc. 2016; 30:245–250.
Article6. Katai H, Sasako M, Fukuda H, Nakamura K, Hiki N, Saka M, et al. Safety and feasibility of laparoscopy-assisted distal gastrectomy with suprapancreatic nodal dissection for clinical stage I gastric cancer: a multicenter phase II trial (JCOG 0703). Gastric Cancer. 2010; 13:238–244.
Article7. Fujita T, Ohta M, Ozaki Y, Takahashi Y, Miyazaki S, Harada T, et al. Collateral thermal damage to the pancreas by ultrasonic instruments during lymph node dissection in laparoscopic gastrectomy. Asian J Endosc Surg. 2015; 8:281–288.
Article8. Obama K, Okabe H, Hosogi H, Tanaka E, Itami A, Sakai Y. Feasibility of laparoscopic gastrectomy with radical lymph node dissection for gastric cancer: from a viewpoint of pancreas-related complications. Surgery. 2011; 149:15–21.
Article9. Hiki N, Honda M, Etoh T, Yoshida K, Kodera Y, Kakeji Y, et al. Higher incidence of pancreatic fistula in laparoscopic gastrectomy. Real-world evidence from a nationwide prospective cohort study. Gastric Cancer. 2018; 21:162–170.
Article10. Debi U, Kaur R, Prasad KK, Sinha SK, Sinha A, Singh K. Pancreatic trauma: a concise review. World J Gastroenterol. 2013; 19:9003–9011.
Article11. Kumagai K, Hiki N, Nunobe S, Kamiya S, Tsujiura M, Ida S, et al. Impact of anatomical position of the pancreas on postoperative complications and drain amylase concentrations after laparoscopic distal gastrectomy for gastric cancer. Surg Endosc. Forthcoming. 2018.
Article12. Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010; 8:e1000412.
Article13. Urano Y, Asanuma D, Hama Y, Koyama Y, Barrett T, Kamiya M, et al. Selective molecular imaging of viable cancer cells with pH-activatable fluorescence probes. Nat Med. 2009; 15:104–109.
Article14. Yamashita S, Sakabe M, Ishizawa T, Hasegawa K, Urano Y, Kokudo N. Visualization of the leakage of pancreatic juice using a chymotrypsin-activated fluorescent probe. Br J Surg. 2013; 100:1220–1228.
Article15. Mori K, Ishizawa T, Yamashita S, Kamiya M, Urano Y, Kokudo N. Intraoperative visualization of pancreatic juice leaking from the pancreatic stump in a swine model. Gastroenterology. 2015; 149:1334–1336.16. Kono Y, Ishizawa T, Tani K, Harada N, Kaneko J, Saiura A, et al. Techniques of fluorescence cholangiography during laparoscopic cholecystectomy for better delineation of the bile duct anatomy. Medicine (Baltimore). 2015; 94:e1005.
Article17. Kudo H, Ishizawa T, Tani K, Harada N, Ichida A, Shimizu A, et al. Visualization of subcapsular hepatic malignancy by indocyanine-green fluorescence imaging during laparoscopic hepatectomy. Surg Endosc. 2014; 28:2504–2508.
Article18. Moore EE, Cogbill TH, Malangoni MA, Jurkovich GJ, Champion HR, Gennarelli TA, et al. Organ injury scaling, II: pancreas, duodenum, small bowel, colon, and rectum. J Trauma. 1990; 30:1427–1429.19. Tsujiura M, Hiki N, Ohashi M, Nunobe S, Kumagai K, Ida S, et al. “Pancreas-compressionless gastrectomy”: a novel laparoscopic approach for suprapancreatic lymph node dissection. Ann Surg Oncol. 2017; 24:3331–3337.
Article20. Sano T, Sasako M, Katai H, Maruyama K. Amylase concentration of drainage fluid after total gastrectomy. Br J Surg. 1997; 84:1310–1312.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Is Laparoscopic Approach Also Safe for the Treatment of Remnant Gastric Cancer?
- Pure single-incision laparoscopic D2 lymphadenectomy for gastric cancer: a novel approach to 11p lymph node dissection (midpancreas mobilization)
- Gastric cancer in pregnancy: is laparoscopic gastrectomy with lymph node dissection feasible and safe?
- Chyle Leakage After Right Selective Lymph Node Dissection in a Patient With Papillary Thyroid Cancer: A Case Report
- Totally Laparoscopic Pylorus-preserving Gastrectomy with D2 Lymph Node Dissection